
Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ ASRM-Sponsored 3rd PCOS Consensus Workshop Group
Download a PDF of this document
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in females, with a high prevalence. The etiology of this heterogeneous condition remains obscure, and its phenotype expression varies. Two widely cited previous ESHRE/ASRM sponsored PCOS consensus workshops focused on diagnosis (published in 2004) and infertility management (published in 2008), respectively. The present third PCOS consensus report summarizes current knowledge and identifies knowledge gaps regarding various women’s health aspects of PCOS. Relevant topics addressed—all dealt with in a systematic fashion—include adolescence, hirsutism and acne, contraception, menstrual cycle abnormalities, quality of life, ethnicity, pregnancy complications, long-term metabolic and cardiovascular health, and finally cancer risk. Additional, comprehensive background information is provided separately in an extended online publication. (Fertil Steril® 2012;97:28–38. ©2012 by American Society for Reproductive Medicine.)
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women, with a prevalence between 6% and 10% based on the U.S. National Institutes of Health (NIH) criteria and as high as 15% when the broader Rotterdam criteria are applied. Typically, PCOS is first identified during the early reproductive years. The clinical expression varies but commonly includes oligo ovulation or anovulation, hyperandrogenism (either clinical or biochemical), and the presence of polycystic ovaries. The etiology of the syndrome remains obscure, and the variability in phenotype expression continues to render the clinical care and research concerning this heterogeneous condition challenging.
Two ESHRE/ASRM-sponsored PCOS consensus workshops have previously been organized. The first one in Rotterdam, the Netherlands, in 2003 focused on diagnostic criteria for PCOS (1, 2); the second in Thessaloniki, Greece, in 2007 dealt with infertility management in PCOS (3, 4). The conclusions of these meetings were subsequently jointly published simultaneously in both Human Reproduction and Fertility and Sterility. These papers are highly cited, suggesting a great interest in this area and underlining the value of such consensus contributions.
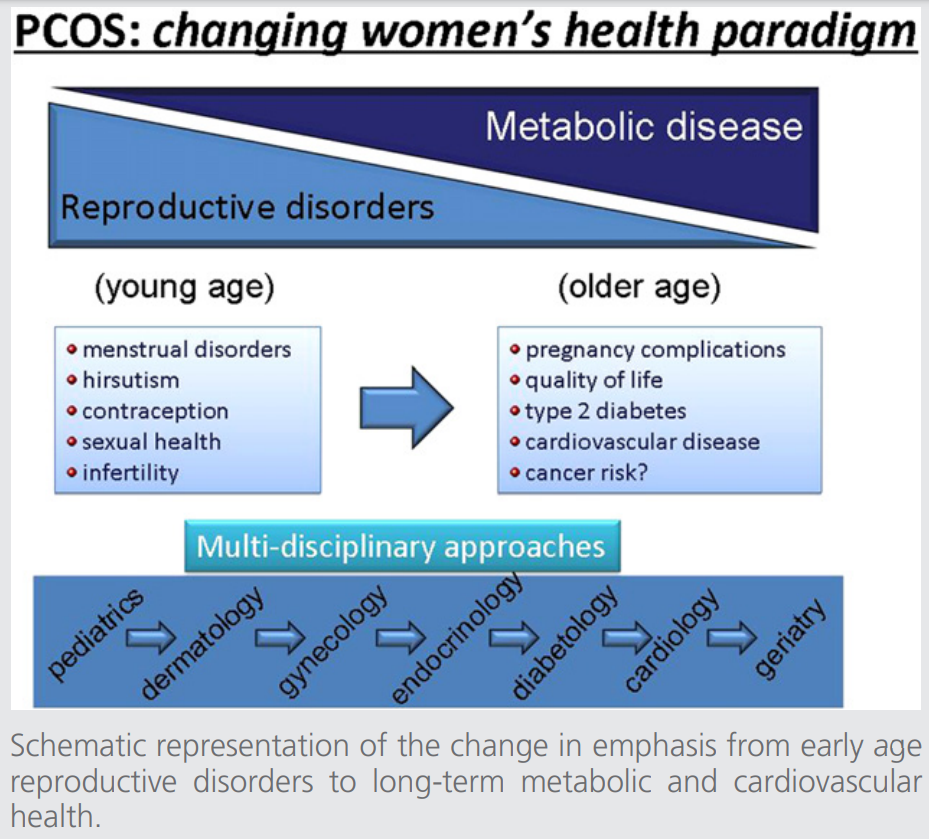
A third PCOS consensus workshop—the focus of the present report—took place in Amsterdam, the Netherlands, in October 2010 and attempted to summarize current knowledge and to identify gaps in knowledge regarding various women’s health aspects of PCOS. Diverse aspects of care during the reproductive and postreproductive years were addressed, including adolescence, hirsutism and acne, contraception, menstrual cycle abnormalities, quality of life and sexual health, ethnicity, pregnancy complications, long-term (metabolic) cardiovascular health, and cancer risk (Fig. 1). Due to the complexity of the many issues discussed, this contribution will address each topic separately in a fixed format: a brief introduction, concluding statements (where there was agreement), a summary of areas of disagreement (if any) and knowledge gaps, with recommended directions for future research. These concluding statements in relation to each specific topic mentioned are published in the journals (maximum of 5 references per paragraph). An extended version of this article is available online with Supplemental Material that provides comprehensive background information.
The hierarchy of the evidence available in the literature assessed for this conference was graded as follows:
Level A requires at least one randomized, controlled trial (RCT) as part of a body of literature of overall good quality and consistency that addresses the specific recommendation.
Level B requires the availability of well-controlled clinical studies, but no RCTs on the topics of recommendation.
Level C requires evidence obtained from expert committee reports of opinions and/or clinical experiences of respected authorities, which indicates an absence of directly applicable clinical studies of good quality.
Good practice points (GPP) are also addressed.
There is no overall agreement as to how to diagnose PCOS in adolescence. Acne is common during the adolescent years, whether or not PCOS is present, whereas hirsutism—associated with PCOS—typically develops over time. Hyperandrogenemia may be a more consistent marker for PCOS during the teenage years (5). In all young women, irregular menses are common in the years immediately after menarche. As many as 85% of menstrual cycles are anovulatory during the first year after menarche, and up to 59% are still anovulatory during the third year after menarche (6). In one study, persisting oligomenorrhea was not predicted by increased androgens, polycystic ovaries on ultrasound, or increased serum luteinizing hormone (LH) levels (7). Increased body mass index (BMI), however, was the major risk factor for persistent anovulation. Only approximately 40% of adolescent women with menstrual irregularity have polycystic ovaries on ultrasound (8). These considerations have led to the suggestion that all three elements of the Rotterdam criteria should be present in teenagers to make the diagnosis of PCOS (9). These investigators suggest that oligomenorrhea or amenorrhea should be present for at least 2 years after menarche (or primary amenorrhea at age 16 yrs), the diagnosis of polycystic ovaries on ultrasound should include increased ovarian size (>10 cm3), and hyperandrogenemia rather than just signs of androgen excess should be documented.
Hirsutism is a good marker for hyperandrogenism even when considering ethnic differences and systemic factors such as obesity. Hirsutism is present in approximately 70% of women with PCOS, but hyperandrogenemia should be evaluated biochemically in all women suspected of having PCOS. By comparison, acne and alopecia are not commonly associated with hyperandrogenemia and therefore should not be regarded as evidence of hyperandrogenemia.
For women with PCOS in whom hirsutism is a major concern, treatment is focused on reduction of androgen production, decreasing the fraction of circulating free testosterone (T), and limiting androgen bioactivity to hair follicles. In those women with PCOS who have acne vulgaris, clinical benefit may be derived from many systemic therapeutic modalities. Because terminal hair turnover occurs slowly, at least 6 months of treatment is generally considered the minimal interval to see a response.
The main therapeutic emphasis has focused on inhibition of ovarian steroid production and decreased bioavailability through augmentation of sex hormone binding globulin (SHBG) levels with the use of oral contraceptive pills (OCPs). Often OCPs are prescribed in combination with an antiandrogen to block androgen action at the hair follicles. Antiandrogens include spironolactone (an aldosterone-antagonist diuretic), flutamide (an androgen receptor antagonist), and finasteride (a 5a-reductase type 2 inhibitor). In general, the addition of an antiandrogen to OCPs has not appeared to increase the overall treatment benefit. Each of these agents have been shown to reduce hirsutism, and all appear (without head-to-head comparisons) to have equivalent efficacy (10–12). Notably, antiandrogens should not be used without effective contraception (given their potential fetal toxicity). Flutamide is of limited value because of associated hepatotoxicity. In addition, drospirenone is not antiandrogenic in the dosage used as a component of some OCPs. Insulin-sensitizing agents, such as metformin and pioglitazone, have little effect on hirsutism or acne (13, 14). Physical approaches to remove unwanted hair, including electrolysis and laser treatments, may be acceptable to many patients.
In severe acne, isotretinoin can be beneficial, but individual responses vary. It is not effective for hirsutism and occasionally may lead to alopecia. Physical approaches to remove unwanted hair, including electrolysis and laser treatments, may be acceptable to many patients. Topical treatment with eflornithine hydrochloride, an inhibitor of ornithine decarboxylase, limits cell division and has been shown to be effective for decreasing the development of new unwanted facial hair (15). No effective pharmacologic treatment for alopecia exists.
Although cycle abnormalities are common during the reproductive years, women with PCOS may ovulate spontaneously. How frequently this occurs is unknown (16), but ovulations have been reported in up to 32% of ‘‘cycles.’’ Women with oligomenorrhea or amenorrhea have about a 90% chance of being diagnosed with PCOS, and up to 95% of affected adults have oligomenorrhea or amenorrhea (17). The definition used to establish the diagnosis of PCOS affects the proportion of women included with menstrual irregularities (18).
Amenorrheic women with PCOS usually have the most severe hyperandrogenism and higher antral follicle counts as compared with women presenting with oligomenorrhea or regular menstrual cycles. Menstrual cycles in women with PCOS become more regular as they approach menopause (19, 20). Obesity rather than the menstrual cycle pattern or the size of the follicular cohort determines hyperinsulinemia, dyslipidemia, and hypertension in aging women with PCOS (20).
Women with PCOS who do not desire pregnancy need contraception. No contraceptive methods are contraindicated in PCOS. However, some of the features associated with PCOS (such as obesity and insulin resistance) may represent a relative contraindication to the use of combined OCPs. Cycle control is usually achieved by the use of OCP in women with PCOS.
Oral contraceptives suppress LH secretion and lead to a decrease in ovarian androgen production. The estrogenic component increases the levels of sex hormone–binding globulin (SHBG), which, in turn, results in a decrease in circulating free T levels. The progestin in the pill can compete for 5a-reductase at the level of the androgen receptor. Oral contraception also decreases adrenal androgen production by a mechanism yet unclear, possibly due to a decrease in adrenocorticotropin hormone (ACTH) production.
There are few randomized double-blind studies comparing the metabolic effects of a combination of two OCPs, or combined with an insulin sensitizer (21). A Cochrane review, based on limited evidence, concluded that OCP use does not increase metabolic risk (22). Findings from a few small studies suggest that insulin resistance worsens during the natural course of PCOS, but long-term OCP use either does not change or improves cardiometabolic risk parameters, including insulin resistance, lipoprotein profile, and possibly body fat distribution.
Patients with PCOS are an at-risk group for psychological and behavioral disorders and reduced quality-of-life (QOL) (23–25). Studies in this area have been hampered by the existence of only one validated disease-specific questionnaire, the QOL Questionnaire for Women with PCOS (PCOSQ) (26). A review of generic and specific quality-of-life studies in women with PCOS concluded that (1) PCOS has a significant detrimental effect on QOL compared with controls, (2) weight issues are most apt to affect quality of life, (3) few studies include an instrument specific for PCOS in their assessment, and (4) very few studies include QOL instruments in their assessment of the benefits of the investigated treatment (24).
The PCOSQ cannot be used to evaluate the prevalence of emotional and other disorders (e.g., sexual or eating disorders). However, from other validated measures, it appears that patients with PCOS are at higher risk for developing significant psychological difficulties (i.e., depression, anxiety) compared with healthy and other controls and may also be at risk for eating disorders and sexual and relational dysfunction, though this evidence is inconsistent (23). It has been suggested that women with PCOS should undergo psychological screening to improve their long-term prognosis. However, until it is possible to disentangle potential features of the disorder from reactions to it, recommending psychological screening is premature.
Two ESHRE/ASRM-sponsored PCOS consensus workshops have previously been organized. The first one in Rotterdam, the Netherlands, in 2003 focused on diagnostic criteria for PCOS (1, 2); the second in Thessaloniki, Greece, in 2007 dealt with infertility management in PCOS (3, 4). The conclusions of these meetings were subsequently jointly published simultaneously in both Human Reproduction and Fertility and Sterility. These papers are highly cited, suggesting a great interest in this area and underlining the value of such consensus contributions.
A third PCOS consensus workshop—the focus of the present report—took place in Amsterdam, the Netherlands, in October 2010 and attempted to summarize current knowledge and to identify gaps in knowledge regarding various women’s health aspects of PCOS. Diverse aspects of care during the reproductive and postreproductive years were addressed, including adolescence, hirsutism and acne, contraception, menstrual cycle abnormalities, quality of life and sexual health, ethnicity, pregnancy complications, long-term (metabolic) cardiovascular health, and cancer risk (Fig. 1). Due to the complexity of the many issues discussed, this contribution will address each topic separately in a fixed format: a brief introduction, concluding statements (where there was agreement), a summary of areas of disagreement (if any) and knowledge gaps, with recommended directions for future research. These concluding statements in relation to each specific topic mentioned are published in the journals (maximum of 5 references per paragraph). An extended version of this article is available online with Supplemental Material that provides comprehensive background information.
The hierarchy of the evidence available in the literature assessed for this conference was graded as follows:
Level A requires at least one randomized, controlled trial (RCT) as part of a body of literature of overall good quality and consistency that addresses the specific recommendation.
Level B requires the availability of well-controlled clinical studies, but no RCTs on the topics of recommendation.
Level C requires evidence obtained from expert committee reports of opinions and/or clinical experiences of respected authorities, which indicates an absence of directly applicable clinical studies of good quality.
Good practice points (GPP) are also addressed.
ADOLESCENCE
There is no overall agreement as to how to diagnose PCOS in adolescence. Acne is common during the adolescent years, whether or not PCOS is present, whereas hirsutism—associated with PCOS—typically develops over time. Hyperandrogenemia may be a more consistent marker for PCOS during the teenage years (5). In all young women, irregular menses are common in the years immediately after menarche. As many as 85% of menstrual cycles are anovulatory during the first year after menarche, and up to 59% are still anovulatory during the third year after menarche (6). In one study, persisting oligomenorrhea was not predicted by increased androgens, polycystic ovaries on ultrasound, or increased serum luteinizing hormone (LH) levels (7). Increased body mass index (BMI), however, was the major risk factor for persistent anovulation. Only approximately 40% of adolescent women with menstrual irregularity have polycystic ovaries on ultrasound (8). These considerations have led to the suggestion that all three elements of the Rotterdam criteria should be present in teenagers to make the diagnosis of PCOS (9). These investigators suggest that oligomenorrhea or amenorrhea should be present for at least 2 years after menarche (or primary amenorrhea at age 16 yrs), the diagnosis of polycystic ovaries on ultrasound should include increased ovarian size (>10 cm3), and hyperandrogenemia rather than just signs of androgen excess should be documented.
Conclusions (Agreement)
- Criteria for the diagnosis of PCOS in adolescents differ from those used for older women of reproductive age (level B).
- Groups at risk (e.g., obese, hirsute, irregular menses) should be identified, but physicians should be cautious of overdiagnosing PCOS (level B).
- Individual PCOS manifestations in adolescents (e.g., obesity, hirsutism, irregular menses) (level B) should be treated.
Knowledge Gaps/Recommended Future Research
- Absence of longitudinal studies through adolescence.
- Absence of specific diagnostic criteria for identifying PCOS early in adolescence.
- Absence of normative values for a number of biochemical markers during adolescence.
- Assessment of value of intervention in PCOS early in adolescence.
- Lack of clarity as to whether the severity of symptoms during adolescence predicts the extent of the disorder in later life.
HIRSUTISM/ACNE/ALOPECIA
Hirsutism is a good marker for hyperandrogenism even when considering ethnic differences and systemic factors such as obesity. Hirsutism is present in approximately 70% of women with PCOS, but hyperandrogenemia should be evaluated biochemically in all women suspected of having PCOS. By comparison, acne and alopecia are not commonly associated with hyperandrogenemia and therefore should not be regarded as evidence of hyperandrogenemia.For women with PCOS in whom hirsutism is a major concern, treatment is focused on reduction of androgen production, decreasing the fraction of circulating free testosterone (T), and limiting androgen bioactivity to hair follicles. In those women with PCOS who have acne vulgaris, clinical benefit may be derived from many systemic therapeutic modalities. Because terminal hair turnover occurs slowly, at least 6 months of treatment is generally considered the minimal interval to see a response.
The main therapeutic emphasis has focused on inhibition of ovarian steroid production and decreased bioavailability through augmentation of sex hormone binding globulin (SHBG) levels with the use of oral contraceptive pills (OCPs). Often OCPs are prescribed in combination with an antiandrogen to block androgen action at the hair follicles. Antiandrogens include spironolactone (an aldosterone-antagonist diuretic), flutamide (an androgen receptor antagonist), and finasteride (a 5a-reductase type 2 inhibitor). In general, the addition of an antiandrogen to OCPs has not appeared to increase the overall treatment benefit. Each of these agents have been shown to reduce hirsutism, and all appear (without head-to-head comparisons) to have equivalent efficacy (10–12). Notably, antiandrogens should not be used without effective contraception (given their potential fetal toxicity). Flutamide is of limited value because of associated hepatotoxicity. In addition, drospirenone is not antiandrogenic in the dosage used as a component of some OCPs. Insulin-sensitizing agents, such as metformin and pioglitazone, have little effect on hirsutism or acne (13, 14). Physical approaches to remove unwanted hair, including electrolysis and laser treatments, may be acceptable to many patients.
In severe acne, isotretinoin can be beneficial, but individual responses vary. It is not effective for hirsutism and occasionally may lead to alopecia. Physical approaches to remove unwanted hair, including electrolysis and laser treatments, may be acceptable to many patients. Topical treatment with eflornithine hydrochloride, an inhibitor of ornithine decarboxylase, limits cell division and has been shown to be effective for decreasing the development of new unwanted facial hair (15). No effective pharmacologic treatment for alopecia exists.
Conclusions (Agreement)
- Hirsutism, considering ethnic differences, is a good marker for hyperandrogenism (level B).
- Isolated acne and alopecia are not necessarily related to and are not good markers for hyperandrogenism (level B).
- Hirsutism should be evaluated biochemically (level B).
- Prolonged (>6 months) medical therapy for hirsutism is necessary to document effectiveness (level B).
- Many drugs used for the treatment of hirsutism are not approved by the U.S. Food and Drug Administration (FDA) for this indication (GPP).
- No effective treatment for alopecia is known (level B).
- Antiandrogens should not be used without effective contraception (level B).
- Flutamide is of limited value because of its dose-dependent hepatotoxicity (level B).
- Drospirenone in the dosage used in some OCPs is not antiandrogenic (level B).
Knowledge Gaps/Recommended Future Research
- Unclear what is the best medical therapy for hirsutism.
- Unclear how long therapy should be continued.
- Unclear how best to evaluate hirsutism clinically.
- Measurement of serum androgens fraught with error but the best estimate we have for hyperandrogenism.
MENSTRUAL IRREGULARITY
Although cycle abnormalities are common during the reproductive years, women with PCOS may ovulate spontaneously. How frequently this occurs is unknown (16), but ovulations have been reported in up to 32% of ‘‘cycles.’’ Women with oligomenorrhea or amenorrhea have about a 90% chance of being diagnosed with PCOS, and up to 95% of affected adults have oligomenorrhea or amenorrhea (17). The definition used to establish the diagnosis of PCOS affects the proportion of women included with menstrual irregularities (18). Amenorrheic women with PCOS usually have the most severe hyperandrogenism and higher antral follicle counts as compared with women presenting with oligomenorrhea or regular menstrual cycles. Menstrual cycles in women with PCOS become more regular as they approach menopause (19, 20). Obesity rather than the menstrual cycle pattern or the size of the follicular cohort determines hyperinsulinemia, dyslipidemia, and hypertension in aging women with PCOS (20).
Conclusions (Agreement)
- Both amenorrheic and oligomenorrheic women may occasionally ovulate (level B).
- Menstrual cycles in women with PCOS may become more regular later in life (level B).
- Irregular menses are associated with increased metabolic risk (level B).
- The greater the menstrual irregularity, the more severe the PCOS phenotype (level B).
Disagreement
- The time needed before regular menstrual cycles occur in young women.
- The extent to which irregular menses (especially amenorrhea) are a source of psychological morbidity and/or decreased quality of life.
Knowledge Gaps/Recommended Future Research
- It is unclear to what extent the severity of the menstrual disturbance is associated with the severity of the PCOS phenotype.
- The natural history and progression of menstrual irregularity in PCOS are not well understood.
- It remains unclear whether PCOS patients have a longer reproductive life span.
- How often do oligomenorrheic or amenorrheic women ovulate?
CONTRACEPTION
Women with PCOS who do not desire pregnancy need contraception. No contraceptive methods are contraindicated in PCOS. However, some of the features associated with PCOS (such as obesity and insulin resistance) may represent a relative contraindication to the use of combined OCPs. Cycle control is usually achieved by the use of OCP in women with PCOS.Oral contraceptives suppress LH secretion and lead to a decrease in ovarian androgen production. The estrogenic component increases the levels of sex hormone–binding globulin (SHBG), which, in turn, results in a decrease in circulating free T levels. The progestin in the pill can compete for 5a-reductase at the level of the androgen receptor. Oral contraception also decreases adrenal androgen production by a mechanism yet unclear, possibly due to a decrease in adrenocorticotropin hormone (ACTH) production.
There are few randomized double-blind studies comparing the metabolic effects of a combination of two OCPs, or combined with an insulin sensitizer (21). A Cochrane review, based on limited evidence, concluded that OCP use does not increase metabolic risk (22). Findings from a few small studies suggest that insulin resistance worsens during the natural course of PCOS, but long-term OCP use either does not change or improves cardiometabolic risk parameters, including insulin resistance, lipoprotein profile, and possibly body fat distribution.
Conclusions (Agreement)
- Overall, the benefits of OCPs outweigh the risks in most patients with PCOS (level B).
- Women with PCOS are more likely to have contraindications for OCP use than normal women (level C).
- In the absence of other risk factors, there is no evidence that women with PCOS are at increased risk with OCPs compared with normal women (level C).
- There is no evidence for differences in effectiveness and risk among the various progestogens and when used in combination with a 20 versus a 30 µg daily dose of estrogen (level B).
- Subsequent fertility is not negatively affected by OCPs (level C).
- There is no definitive evidence that the type of OCP determines efficacy of hirsutism control (level C).
Knowledge Gaps/Recommended Future Research
- Head-to-head blinded trials comparing different OCP strategies are lacking.
- There is a lack of longitudinal follow-up studies after a course of OCPs.
QUALITY OF LIFE
Patients with PCOS are an at-risk group for psychological and behavioral disorders and reduced quality-of-life (QOL) (23–25). Studies in this area have been hampered by the existence of only one validated disease-specific questionnaire, the QOL Questionnaire for Women with PCOS (PCOSQ) (26). A review of generic and specific quality-of-life studies in women with PCOS concluded that (1) PCOS has a significant detrimental effect on QOL compared with controls, (2) weight issues are most apt to affect quality of life, (3) few studies include an instrument specific for PCOS in their assessment, and (4) very few studies include QOL instruments in their assessment of the benefits of the investigated treatment (24).The PCOSQ cannot be used to evaluate the prevalence of emotional and other disorders (e.g., sexual or eating disorders). However, from other validated measures, it appears that patients with PCOS are at higher risk for developing significant psychological difficulties (i.e., depression, anxiety) compared with healthy and other controls and may also be at risk for eating disorders and sexual and relational dysfunction, though this evidence is inconsistent (23). It has been suggested that women with PCOS should undergo psychological screening to improve their long-term prognosis. However, until it is possible to disentangle potential features of the disorder from reactions to it, recommending psychological screening is premature.
Figure 1
Conclusions (Agreement)
- There is evidence of increased prevalence of psychological disorders in women with PCOS (level B).
- Psychological issues should be considered in all women with PCOS because of evidence suggesting increased prevalence and associated comorbidities (level C).
- It is unclear if this increased prevalence is due to the disorder itself or its manifestations (e.g., obesity, hirsutism, irregular menses, infertility) (level C).
- Based on the consultation and the patient’s perception of her problems, appropriate counseling and intervention should be offered (level C).
Knowledge Gaps/Recommended Future Research
- Evaluation of the validity of existing instruments for psychopathology as screening tools in PCOS.
- Determination of the prevalence of psychological disorders using appropriate instruments.
- Development of appropriate screening instruments and interventions (level C).
- Determination whether disease, its manifestations, or its consequences lead to psychological disorders.
PREGNANCY
Women with PCOS may be subfertile. This may be explained by the effects of obesity and/or metabolic, inflammatory, and endocrine abnormalities on ovulatory function, oocyte quality, and endometrial receptivity. Ovarian hyperandrogenism and hyperinsulinemia may promote premature granulosa cell luteinization, and paracrine dysregulation of growth factors may disrupt the intrafollicular environment and impair cytoplasmic and/or nuclear maturation of oocytes (27). These features are not universal, and oocyte quality, fertilization, and implantation rates in an individual woman with PCOS can be normal (28).During early pregnancy, the embryo may be exposed to androgen excess in utero. This may have long-term effects, particularly on female offspring. Fetal hyperandrogenism may disturb epigenetic programming, in particular those genes regulating reproduction and metabolism. Data in relation to the risk of miscarriage in women with PCOS are conflicting, although miscarriage rates are generally thought to be comparable with other subfertile populations (29, 30). When pregnancy occurs in women with PCOS, there is a higher incidence of gestational diabetes (GDM) (40% to 50%) and associated fetal macrosomia, gestational hypertensive disorders (such as preeclampsia and pregnancy-induced hypertension) (5%), and birth of small-for-gestational-age (SGA) babies (10% to 15%) (31). The use of metformin for women with anovulatory PCOS has no benefit with respect to enhancing either fertility or live-birth rates, and its routine use is not recommended.
Conclusions (Agreement)
- Women with PCOS who desire a pregnancy may be at increased risk for adverse pregnancy outcomes, and this may be exacerbated by obesity and/or insulin resistance (level B).
- Health should be optimized before conception, with advice about smoking cessation, lifestyle, diet, and appropriate vitamin supplementation (e.g., folic acid) (GPP).
- Miscarriage rates are not increased in natural conceptions in women with PCOS, independent of obesity. Miscarriage rates after induction of ovulation mirror those found in other infertile populations (level A).
- Women with PCOS should be observed closely during pregnancy as they may be at increased risk for the development of GDM, gestational hypertension, and associated complications (level B).
- Pregnancy-associated risks are greater in women diagnosed by more classic (NIH) criteria as opposed to nonhyperandrogenic women (level B).
- Babies born from women with PCOS may have increased morbidity and mortality (level B).
- There is no evidence for improved live-birth rates or decreased pregnancy complications with the use of metformin either before conception or during pregnancy (level A).
Knowledge Gaps/Recommended Future Directions for Research
- Is there any value to specific periconceptional diets for women with PCOS?
- Should pregnancies of women with PCOS have increased antenatal monitoring, including earlier screening for GDM and additional Doppler studies?
- What is the long-term outcome of children born from women with PCOS?
- What is the long-term outcome for women with PCOS who develop gestational hypertension and GDM compared with women with PCOS who do not conceive?
ETHNIC DIFFERENCES IN THE PHENOTYPE
There is considerable ethnic variation in the expression of PCOS, including the prevalence and severity of obesity, metabolic disturbances, and their correlates. There are differences in psychosocial aspects affecting QOL and health-seeking behaviors (32). For example, Asian women are generally shorter, have a lower BMI, and a milder hyperandrogenic phenotype. South Asians in particular have a high prevalence of the metabolic syndrome (MetS) and are at risk for type 2 diabetes (T2D), with central obesity more than BMI reflecting their metabolic risk (33). A common clinical indicator of greater metabolic risk is acanthosis nigricans.African American and Hispanic women are more often obese and more prone to metabolic problems; women of African descent are particularly prone to hypertension and cardiovascular disease, whereas Hispanic women are more at risk for MetS and T2D (34). There is a strikingly high prevalence of hirsutism among women of Middle Eastern and Mediterranean origin. Nevertheless, abnormal glucose tolerance in southern and eastern Europeans is far less common than in South Asians and Hispanics (33, 35). Geographic location, ethnic origin, and cultural/social practices are likely contributors to the differing manifestations of PCOS and should be recognized in routine clinical practice.
Conclusions (Agreement)
- Ethnic origin and culture contribute to the differing manifestations of PCOS (level B).
- Ethnically appropriate thresholds are required for identifying anthropometric cutoffs for appropriate metabolic screening in high-risk ethnic groups (level B).
Knowledge Gaps/Future Directions for Research
- Effects of migration and rapid economic development for different ethnic groups for long-term cardiovascular and metabolic risk.
- Population-based prevalence of PCOS in all ethnicities.
- Best management for manifestations by ethnicity, and the role of genetic and environmental factors to explain ethnic variances.
- Effects of insulin sensitizers in different ethnic groups.
OBESITY
There is widespread variability in the prevalence of overweight (BMI 25 to 30 kg/m2) and obese (BMI >30 kg/m2) women in PCOS populations across different countries. The proportion of women with PCOS who are overweight but not obese ranges from 10% in Italy to 37% in Kuwait. The highest prevalence of obesity is reported in studies conducted in the United States and Australia, with 61% to 76% of women with PCOS considered obese (36, 37).Women with PCOS are more likely to have upper-body fat distribution compared with weight-matched controls. Greater abdominal or visceral adiposity is associated with greater insulin resistance, which could exacerbate the reproductive and metabolic abnormalities in PCOS (38). It is known that obesity is associated with PCOS, but its causal role in this condition has yet to be determined. Very few studies report the association of BMI with menstrual irregularity. Few randomized controlled studies have been performed on lifestyle interventions, but these suggest substantial reproductive and metabolic benefits (39, 40).
Conclusions (Agreement)
- The prevalence of obesity is increasing and has an important bearing on the phenotype of PCOS (level B).
- Some studies suggest that higher BMI is associated with a greater prevalence of menstrual irregularity, hyperandrogenemia, and hirsutism, but more studies are required to confirm this (level B).
- Increased BMI and visceral adiposity are associated with greater insulin resistance as in the general population, but its effect on menstrual irregularity and hirsutism remain unclear (level B).
- Lifestyle management results in weight loss and improves surrogate markers of metabolic disease/syndrome (level A).
Knowledge Gaps/Recommended Future Directions for Research
- Mechanistic studies are necessary to understand the evolution of obesity and PCOS. Does PCOS predispose to obesity, and does obesity unmask latent PCOS?
- More studies are required of the type and duration of exercise specifically for women with PCOS.
- Further research is required on determinants of increasing participation and compliance in lifestyle programs as well as the effects of these interventions on primary outcomes such as live birth, perinatal morbidity, and diabetes prevention.
- Research is required on the role of bariatric surgery for all aspects of PCOS and on the offspring of women with PCOS conceived after such surgery.
- Research is required to optimize lifestyle interventions, maximizing weight loss and minimizing dropouts among participating women.
INSULIN RESISTANCE AND THE METABOLIC SYNDROME (METS)
Insulin resistance is a prevalent finding in the obese in general, and in women with PCOS (41). It is most prevalent and severe in those with the classic NIH PCOS phenotype involving hyperandrogenism and chronic anovulation. Women with PCOS assessed by the Rotterdam criteria yet with regular cycles are metabolically less abnormal (40, 42, 43).The cellular and molecular mechanisms of insulin resistance in PCOS differ from those in other common insulin-resistant states such as obesity and T2D. In vivo insulin action is profoundly decreased in skeletal muscle secondary to signaling defects, but hepatic insulin resistance is present only in obese women with PCOS. There is a synergistic negative effect of having both PCOS and obesity on insulin action. Pancreatic b-cell dysfunction is also present in PCOS but may be more related to T2D risk factors as this dysfunction is most severe in women with a first-degree relative who has T2D (44).
Extensive evidence indicates that hyperinsulinemia contributes directly to reproductive dysfunction in PCOS (41). Women with classic NIH PCOS have significantly increased rates of the MetS compared with reproductively normal women of similar age and weight.
Conclusions (Agreement)
- Metabolic disorders associated with PCOS are major predictors of prediabetes, diabetes, and MetS in reproductive-age women (level B).
- Patients with MetS are an important clinical subset of women with PCOS (level B).
- Not all PCOS phenotypes have similar metabolic risk. The combination of hyperandrogenemia and oligomenorrhea signifies the most at-risk group (level B).
- It is critical for public health and for optimum design of long-term studies to stratify women with PCOS according to metabolic risk. This goal would be greatly facilitated by using a specific name for this high metabolic risk PCOS subset (GPP).
Knowledge Gaps/Recommended Future Directions for Research
- Long-term prospective studies to define metabolic outcomes and cardiovascular disease (CVD) risk in PCOS.
- Research on the role of androgens in the spectrum of MetS risk in women.
- Further definition of the importance of adipocyte pathophysiology, in particular in the visceral adipose depot, in the evolution of insulin resistance and MetS in PCOS.
TYPE 2 DIABETES (T2D)
Insulin resistance is a prominent feature of PCOS. There is now compelling evidence fromepidemiologic data (45) that PCOS is associated with increased risk of impaired glucose tolerance (IGT), GDM, and T2D (31, 40, 41). Biochemical screening in the form of an oral glucose tolerance test (OGTT) is indicated in obese women with PCOS, and/or those with increased visceral adiposity, as measured by waist circumference. Risk of IGT or diabetes is highest in women who have both oligoovulation or anovulation and hyperandrogenism, and the risk is further amplified by obesity (46).Management of women at risk for T2D should include diet and lifestyle improvement as the first-line treatment. Metformin treatment is indicated in those with IGT who do not respond adequately to calorie restriction and lifestyle changes. In those with frank diabetes, metformin is safe and effective whereas there is concern about the use of thiazolidinediones and glucagonlike peptide-1 analogues in women of reproductive age (47).
Conclusions (Agreement)
- PCOS is a major risk factor for developing IGT and T2D (level A).
- Obesity (by amplifying insulin resistance) is an exacerbating factor in the development of IGT and T2D in PCOS (level A).
- The increasing prevalence of obesity in the population suggests that a further increase in diabetes in PCOS is to be expected (level B).
- Screening for IGT and T2D should be performed by OGTT (75 g, 0- and 2-hour values). There is no utility for measuring insulin in most cases (level C).
- Screening should be performed in the following conditions: hyperandrogenism with anovulation, acanthosis nigricans, obesity (BMI >30 kg/m2, or >25 in Asian populations), in women with a family history of T2D or GDM (level C).
- Diet and lifestyle are first choice for improving fertility and prevention of diabetes (level B).
- Metformin may be used for IGT and T2D (level A). Avoid use of other insulin sensitizing agents such as thiazolidinediones (GPP).
Knowledge Gaps/Recommended Future Research
- Identification of genetic factors contributing to diabetes risk in PCOS.
- Clear definition of the interaction of obesity and body fat distribution with PCOS in development of IGT and T2D.
- Definition of the prevalence of GDM in a large cohort of women with PCOS.
- Collection of good longitudinal data on progression from IGT to T2D.
- Data on efficacy and safety of newer drugs for treatment of T2D in PCOS (including GLP-1 agonists).
- Better assessment of the efficacy of bariatric surgery and its long-term effect.
CARDIOVASCULAR DISEASE MARKERS
Metabolic dysfunction in women with PCOS leads to exaggerated risk for cardiovascular disease (CVD) with aging. Markers for CVD risk reflect the metabolic dysfunction. Changes can occur without obesity and are magnified with obesity. More android central obesity occurs in nonobese women with PCOS. Severity of insulin resistance is related to the amount of abdominal obesity even in women with a normal BMI. This is likely to contribute to the abnormalities in the classic markers for CVD risk (IGT, metabolic syndrome, T2DM, dyslipidemia).The odds for these CVD risk indicators are approximately three times higher in women with PCOS compared with women without PCOS, and in BMI-matched studies the odds are approximately double. The prevalence of these increased CVD risk markers differs by geographic region (48). The more severe PCOS phenotypes are associated with a greater magnitude of CVD risk, and this has been found in obese and nonobese women (49, 25).
Triglyceride, low density lipoprotein (LDL), and non–high density lipoprotein (HDL) cholesterol changes are higher compared with women who do not have PCOS. This reflects more atherogenic apolipoprotein B (ApoB)/ApoA ratios. Differences are greater when PCOS is diagnosed using NIH rather than Rotterdam criteria. Assessing waist circumference and non-HDL-cholesterol appear to be the most useful clinical indicators of this metabolic disturbance. Systemic inflammation associated with endothelial vascular dysfunction and metabolic disturbance is commonly present in women with PCOS. Numerous biochemical inflammatory and thrombotic markers of CVD risk circulate in excess in women with PCOS. Some of these markers correlate with insulin resistance. It remains unclear if increased levels of markers of inflammation and thrombotic risk CVD risk provide additional predictive power beyond assessment using classic CVD risk factor estimates for estimating individual of CVD.
Conclusions (Agreement)
- PCOS at any age is characterized by greater odds for elevated CVD risk markers. Elevated markers occur without obesity and are magnified with obesity (level B).
- Dyslipidemia, IGT, and T2D (classic risk indicators of atherosclerosis and CVD) are more prevalent in women with PCOS, even when weight matched with normal control women (level B).
- Altered levels of triglycerides, HDL, LDL, and non-HDL (reflecting altered ApoB/ApoA metabolism) are prevalent in women with PCOS and are more severe in hyperandrogenic women (level B).
- Non-HDL cholesterol and waist measurement appear to be the best clinical indicators of elevated CVD risk (level C).
- All markers reflect a greater magnitude of risk when women are diagnosed using NIH criteria (including hyperandrogenism) compared with the Rotterdam criteria (level B).
- Depression and anxiety, major risk factors for CVD, are common in women with PCOS (level B).
- The recommended CVD risk assessment at any age is for psychosocial stress, blood pressure, glucose, lipid profile (cholesterol, triglycerides, HDL, LDL, and non-HDL cholesterol), waist circumference, physical activity, nutrition, and smoking (level C).
- Because CVD risk increases with age and accompanying additive environmental insults, periodic reassessment for CVD risk is recommended (GPP).
Knowledge Gaps/Recommended Future Research
- How often should CVD risk assessment be repeated in women with PCOS with or without elevated risk indicators?
- What are optimal specific recommendations in various races or ethnicities?
- Which novel CVD risk markers provide added benefit beyond the classic CVD risk indicators?
- Longitudinal studies associating surrogate markers with CVD events are needed for precise CVD risk prediction.
CARDIOVASCULAR DISEASE OUTCOMES
Life-long metabolic dysfunction in women with PCOS exaggerates the risk for CVD with aging, particularly after menopause. This metabolic dysfunction is based upon insulin resistance, which occurs in most women with PCOS and is independent of and additive with obesity. Consequently, beginning in adolescence, IGT and T2D are highly prevalent in PCOS (odds ratio (OR) of approximately 4:1) and occur in about 40% of women with PCOS by the fourth decade of life, with age and weight gain worsening glycemic control. Insulin-resistant women with PCOS have vascular dysfunction, which is associated with total and abdominal adiposity. Women with PCOS also have more subclinical vascular disease than normal women. The severity of carotid-intima media thickening, coronary artery calcification, and to a lesser extent aortic calcification are greater in women with PCOS (by NIH criteria) than controls, independent of age and BMI.Nevertheless, evidence for increased CVD morbidity and mortality in women with PCOS, based upon Rotterdam and/or NIH criteria, remains inconclusive (20, 50–52). It is not possible to properly diagnose PCOS after menopause. Nevertheless, postmenopausal women with existent hyperandrogenemia and premenopausal menstrual irregularity have a larger number of cardiovascular events than controls, despite technical challenges in accurately measuring low circulating androgen levels in this age group (53). Among nondiabetic postmenopausal women with intact ovaries, moreover, atherosclerotic CVD is associated with features of PCOS, including premenopausal menstrual irregularity, hirsutism, and postmenopausal biochemical hyperandrogenism (54).
Conclusions (Agreement)
- Life-long metabolic dysfunction in women with PCOS exaggerates CVD risk, causing a possible increase in CVD events with age, especially after menopause (level B).
- All surrogate markers of cardiovascular risk are higher in PCOS (adjusted for age and BMI), but the association of these markers with cardiovascular events in PCOS remains unclear (level B).
- Endothelial dysfunction in PCOS is related to abdominal obesity and insulin resistance (level B).
- Coronary artery calcification and carotid intima media wall thickness are also increased in women with PCOS compared with matched controls (level B).
- Among nondiabetic postmenopausal women with intact ovaries, atherosclerotic CVD is associated with features of PCOS, such as relative androgen excess and a recalled history of irregular menses (level B).
Disagreement
- Uncertainty exists as to whether PCOS status per se increases cardiovascular mortality.
Knowledge Gaps/Recommended Future Research
- Data are lacking regarding ethnic and racial differences in the set point for vascular damage associated with PCOS.
- Precision of cardiovascular surrogate markers is unknown.
- Association between cardiovascular surrogate markers and cardiovascular events is unclear.
- Longitudinal studies are needed to associate cardiovascular markers with vascular events.
- Longitudinal studies are lacking regarding the effects of various PCOS phenotypes on cardiovascular events.
- The role of sex steroids on regional adipogenesis and its impact on total and abdominal obesity is uncertain.
- It is uncertain whether PCOS phenotypic expression varies over lifetime and modulates cardiovascular risk.
- It is uncertain whether hyperandrogenemia per se has its own independent effects on atherosclerosis.
- The optimum multifaceted approach to women with PCOS that reduces and prevents CVD has yet to be determined.
CANCER RISK
Polycystic ovary syndrome is a common reproductive disorder resulting in a disruption of normal reproductive physiology. This condition may be associated with increased risk of the development of cancer of the endometrium, ovary, and/or breast, either directly or mediated by its associated reproductive metabolic alterations. There is a small to moderate amount of literature assessing the association of PCOS with the development of cancer of the reproductive organs. Estimates of the strength of association are likely to be sensitive to a number of factors including limitations in the definition of PCOS, limitations in comparison with various populations, and the small number of studies assessing each cancer type (50, 52, 55, 56)
Conclusions (Agreement)
- There are moderate quality data to support that women with PCOS have a 2.7-fold (95% confidence interval [CI], 1.0–7.3) increased risk for endometrial cancer. Most endometrial cancers are well differentiated and have a good prognosis (level B).
- Limited data exist that do not support the conclusion that women with PCOS are at increased risk for ovarian cancer (level B).
- Limited data exist that do not support the conclusion that women with PCOS are at increased risk for breast cancer (level B).
Disagreement
- There is no agreement on the optimal modality or timing of how to monitor women for the presence of endometrial cancer or precursor endometrial changes using ultrasound and/or endometrial biopsy. The decision to assess for the presence of endometrial cancer should be based on clinical factors including length of amenorrhea, presence of abnormal uterine bleeding, thickness and appearance of the endometrium on imaging, and the age of the patient (GPP).
Knowledge Gaps/Recommended Future Research
- There is insufficient evidence to evaluate any association of PCOS with vaginal, vulvar, or cervical cancer.
- Cancer risk with PCOS is difficult to separate from other recognized risk factors such as nulliparity, infertility and its treatment, anovulation, and obesity.
- There is a lack of precision in the estimate of the risk of endometrial cancer in PCOS, especially in subgroups with and without risk factors.
- There is limited confidence in the association of PCOS and ovarian cancer.
- Cancer studies in PCOS should involve more patients, with more clarity on the phenotypic variation in the diagnosis of PCOS.
- Comparison population studies should be conducted and improved.
MENOPAUSE, GENERAL HEALTH
The transition of women with PCOS into menopause and whether there is a specific phenotype for PCOS after menopause is poorly understood. There is evidence that women with PCOS have a larger cohort of primary follicles than age-matched control women before menopause. Serum T levels decrease as women age from the third to fifth decades. Additionally, women with PCOS often develop improved menstrual regularity with age. These factors may all contribute to improvement in reproductive functioning with age before menopause. Menopausal PCOS phenotype is poorly defined. The polycystic ovary criterion is likely not useful after menopause.It is not definitively known what the general health status of postmenopausal women with PCOS is, or what are optimum therapies. It is suspected that women with PCOS who have transitioned through menopause will have increased rates of obesity, diabetes, and cardiovascular events. Most reports
tend to show normal or increased bone mineral density in women with PCOS. The natural history of hirsutism and/or alopecia in postmenopausal women with PCOS is unknown. It is difficult from the existing data to know whether the mortality rate is different in women with PCOS. Retrospective data in women with polycystic ovaries suggest mortality occurs at a similar rate as in the general population and presumably at the same age (19, 57–61). Alternative data suggest they have higher rates of stroke and CVD.
Conclusions (Agreement)
- Age may improve many manifestations of PCOS, including normalizing ovarian size and morphology, T levels, and oligo-ovulation before menopause (level B).
Knowledge Gaps/Recommended Future Research
- There are little data on long-term fecundity and precise age of menopause in women with PCOS.
- The long-term risk for morbidity and mortality among postmenopausal women with a history of PCOS is uncertain.
- There is no established phenotype for PCOS after menopause.
- Most clinical assays are not precise for determining T levels in postmenopausal women.
- Long-term, multicenter cohort studies are needed where the following issues should be assessed:menopausal phenotype, cardiovascular events, cancer, and other causes ofmorbidity/mortality.
- Genomewide association studies should be used to identify new genes/pathways involved in ovarian dysfunction related to age of menopause and polycystic ovaries.
Acknowledgments:
The authors thank Mrs. I. Donner, Ms. Y. R. Griesen, and R. C. Brink for secretarial assistance.
REFERENCES
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004;19:41–7.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25.
- Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril 2008;89:505–22.
- Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod 2008;23:462–77.
- Blank SK, Helm KD, McCartney CR, Marshall JC. Polycystic ovary syndrome in adolescence. Ann NY Acad Sci 2008;1135:76–84.
- Apter D. Endocrine and metabolic abnormalities in adolescents with a PCOS-like condition: consequences for adult reproduction. Trends Endocrinol Metab 1998;9:58–61.
- van Hooff MH, Voorhorst FJ, Kaptein MB, Hirasing RA, Koppenaal C, Schoemaker J. Predictive value of menstrual cycle pattern, body mass index, hormone levels and polycystic ovaries at age 15 years for oligo-amenorrhoea at age 18 years. Hum Reprod 2004;19:383–92.
- Venturoli S, Porcu E, Fabbri R, Pluchinotta V, Ruggeri S, Macrelli S, et al. Longitudinal change of sonographic ovarian aspects and endocrine parameters in irregular cycles of adolescence. Pediatr Res 1995;38:974–80.
- Carmina E, Oberfield SE, Lobo RA. The diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol 2010;203:201–5.
- O’Brien RC, Cooper ME, Murray RM, Seeman E, Thomas AK, Jerums G. Comparison of sequential cyproterone acetate/estrogen versus spironolactone/ oral contraceptive in the treatment of hirsutism. J Clin Endocrinol Metab 1991;72:1008–13.
- Erenus M, Yucelten D, Durmusoglu F, Gurbuz O. Comparison of finasteride versus spironolactone in the treatment of idiopathic hirsutism. Fertil Steril 1997;68:1000–3.
- Moghetti P, Tosi F, Tosti A, Negri C, Misciali C, Perrone F, et al. Comparison of spironolactone, flutamide, and finasteride efficacy in the treatment of hirsutism: a randomized, double blind, placebo-controlled trial. J Clin Endocrinol Metab 2000;85:89–94.
- Harborne L, Fleming R, Lyall H, Sattar N, Norman J. Metformin or antiandrogen in the treatment of hirsutism in polycystic ovary syndrome. J Clin Endocrinol Metab 2003;88:4116–23.
- Cosma M, Swiglo BA, Flynn DN, Kurtz DM, Labella ML, Mullan RJ, et al. Clinical review: insulin sensitizers for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab 2008;93:1135–42.
- Balfour JA, McClellan K. Topical eflornithine. Am J Clin Dermatol 2001;2:197–201.
- Laven JS, Imani B, Eijkemans MJ, Fauser BC. New approach to polycystic ovary syndrome and other forms of anovulatory infertility. Obstet Gynecol Surv 2002;57:755–67.
- Kumarapeli V, Seneviratne RA, Wijeyaratne CN, Yapa RM, Dodampahala SH. A simple screening approach for assessing community prevalence and phenotype of polycystic ovary syndrome in a semi-urban population in Sri Lanka. Am J Epidemiol 2008;168:321–8.
- Vutyavanich T, Khaniyao V, Wongtra-Ngan S, Sreshthaputra O, Sreshthaputra R, Piromlertamorn W. Clinical, endocrine and ultrasonographic features of polycystic ovary syndrome in Thai women. J Obstet Gynaecol Res 2007;33:677–80.
- Dahlgren E, Johansson S, Lindstedt G, Knutsson F, Oden A, Janson PO, et al. Women with polycystic ovary syndrome wedge resected in 1956 to 1965: a long-term follow-up focusing on natural history and circulating hormones. Fertil Steril 1992;57:505–13.
- Elting MW, Korsen TJ, Schoemaker J. Obesity, rather than menstrual cycle pattern or follicle cohort size, determines hyperinsulinaemia, dyslipidaemia and hypertension in ageing women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2001;55:767–76.
- Yildiz BO. Oral contraceptives in polycystic ovary syndrome: risk-benefit assessment. Semin Reprod Med 2008;26:111–20.
- Costello MF, Shrestha B, Eden J, Johnson NP, Sjoblom P. Metformin versus oral contraceptive pill in polycystic ovary syndrome: a Cochrane review. Hum Reprod 2007;22:1200–9.
- Himelein MJ, Thatcher SS. Polycystic ovary syndrome and mental health: a review. Obstet Gynecol Surv 2006;61:723–32.
- Jones GL, Hall JM, Balen AH, Ledger WL. Health-related quality of life measurement in women with polycystic ovary syndrome: a systematic review. Hum Reprod Update 2008;14:15–25.
- Dokras A, Clifton S, Futterweit W, Wild R. Increased risk for abnormal depression scores in women with polycystic ovary syndrome: a systematic review and meta-analysis. Obstet Gynecol 2011;117:145–52.
- Cronin L, Guyatt G, Griffith L, Wong E, Azziz R, Futterweit W, et al. Development of a health-related quality-of-life questionnaire (PCOSQ) forwomenwith polycystic ovary syndrome (PCOS). J Clin EndocrinolMetab 1998;83:1976–87.
- Dumesic DA, Padmanabhan V, Abbott DH. Polycystic ovary syndrome and oocyte developmental competence. Obstet Gynecol Surv 2008;63:39–48.
- Weghofer A, Munne S, Chen S, Barad D, Gleicher N. Lack of association between polycystic ovary syndrome and embryonic aneuploidy. Fertil Steril 2007;88:900–5.
- Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev 2010;1:CD003053.
- Vanky E, Stridsklev S, Heimstad R, Romundstad P, Skogoy K, Kleggetveit O, et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: a randomized, controlled multicenter study. J Clin Endocrinol Metab 2010;95:E448–55.
- BoomsmaCM, EijkemansMJ,Hughes EG, VisserGH, FauserBC,MacklonNS.A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update 2006;12:673–83.
- Goodarzi MO, Quinones MJ, Azziz R, Rotter JI, Hsueh WA, Yang H. Polycystic ovary syndrome in Mexican-Americans: prevalence and association with the severity of insulin resistance. Fertil Steril 2005;84:766–9.
- Wijeyaratne CN, Seneviratne RA, Dahanayake S, Kumarapeli V, Palipane E, Kuruppu N, et al. Phenotype and metabolic profile of South Asian women with polycystic ovary syndrome (PCOS): results of a large database from a specialist endocrine clinic. Hum Reprod 2011;26:202–13.
- Lo JC, Feigenbaum SL, Yang J, Pressman AR, Selby JV, Go AS. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab 2006;91:1357–63.
- Kalra P, Bansal B, Nag P, Singh JK, Gupta RK, Kumar S, et al. Abdominal fat distribution and insulin resistance in Indian women with polycystic ovarian syndrome. Fertil Steril 2009;91:1437–40.
- Glueck CJ, Dharashivkar S, Wang P, Zhu B, Gartside PS, Tracy T, et al. Obesity and extreme obesity, manifest by ages 20–24 years, continuing through 32–41 years in women, should alert physicians to the diagnostic likelihood of polycystic ovary syndrome as a reversible underlying endocrinopathy. Eur J Obstet Gynecol Reprod Biol 2005;122:206–12.
- Ching HL, Burke V, Stuckey BG. Quality of life and psychological morbidity in women with polycystic ovary syndrome: body mass index, age and the provision of patient information are significant modifiers. Clin Endocrinol (Oxf) 2007;66:373–9.
- Lord J, Thomas R, Fox B, Acharya U, Wilkin T. The central issue? Visceral fat mass is a good marker of insulin resistance and metabolic disturbance in women with polycystic ovary syndrome. BJOG 2006;113:1203–9.
- Moran LJ, Pasquali R, Teede HJ, Hoeger KM, Norman RJ. Treatment of obesity in polycystic ovary syndrome: a position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertil Steril 2009;92:1966–82.
- Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2010;16:347–63.
- Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 1997;18:774–800.
- Legro RS, Chiu P, Kunselman AR, Bentley CM, Dodson WC, Dunaif A. Polycystic ovaries are common in women with hyperandrogenic chronic anovulation but do not predict metabolic or reproductive phenotype. J Clin Endocrinol Metab 2005;90:2571–9.
- Johnstone EB, Rosen MP, Neril R, Trevithick D, Sternfeld B, Murphy R, et al. The polycystic ovary post-Rotterdam: a common, age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab 2010;95:4965–72.
- EhrmannDA, Sturis J, ByrneMM, Karrison T, Rosenfield RL, Polonsky KS. Insulin secretory defects in polycystic ovary syndrome: relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. J Clin Invest 1995;96:520–7.
- Solomon CG, Hu FB, Dunaif A, Rich-Edwards J, Willett WC, Hunter DJ, et al. Long or highly irregular menstrual cycles as a marker for risk of type 2 diabetes mellitus. JAMA 2001;286:2421–6.
- Barber TM, Wass JA, McCarthy MI, Franks S. Metabolic characteristics of women with polycystic ovaries and oligo-amenorrhoea but normal androgen levels: implications for the management of polycystic ovary syndrome. Clin Endocrinol (Oxf) 2007;66:513–7.
- Franks S. When should an insulin sensitizing agent be used in the treatment of polycystic ovary syndrome? Clin Endocrinol (Oxf) 2011;74:148–51.
- Chen X, Ni R, Mo Y, Li L, Yang D. Appropriate BMI levels for PCOS patients in Southern China. Hum Reprod 2010;25:1295–302.
- Zhao X, Zhong J, Mo Y, Chen X, Chen Y, Yang D. Association of biochemical hyperandrogenism with type 2 diabetes and obesity in Chinese women with polycystic ovary syndrome. Int J Gynaecol Obstet 2010;108:148–51.
- Pierpoint T, McKeigue PM, Isaacs AJ, Wild SH, Jacobs HS. Mortality of women with polycystic ovary syndrome at long-term follow-up. J Clin Epidemiol 1998;51:581–6.
- Cibula D, Cifkova R, Fanta M, Poledne R, Zivny J, Skibova J. Increased risk of non-insulin dependent diabetes mellitus, arterial hypertension and coronary artery disease in perimenopausal women with a history of the polycystic ovary syndrome. Hum Reprod 2000;15:785–9.
- Wild S, Pierpoint T, Jacobs H, McKeigue P. Long-term consequences of polycystic ovary syndrome: results of a 31 year follow-up study. Hum Fertil (Camb) 2000;3:101–5.
- Shaw LJ, Bairey Merz CN, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, et al. Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health—National Heart, Lung, and Blood Institute sponsored Women’s Ischemia Syndrome Evaluation. J Clin Endocrinol Metab 2008;93:1276–84.
- Krentz AJ, von MD, Barrett-Connor E. Searching for polycystic ovary syndrome in postmenopausal women: evidence of a dose-effect association with prevalent cardiovascular disease. Menopause 2007;14:284–92.
- Schildkraut JM, Schwingl PJ, Bastos E, Evanoff A, Hughes C. Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet Gynecol 1996;88:554–9.
- Chittenden BG, Fullerton G, Maheshwari A, Bhattacharya S. Polycystic ovary syndrome and the risk of gynaecological cancer: a systematic review. Reprod Biomed Online 2009;19:398–405.
- Mulders AG, Laven JS, Eijkemans MJ, de Jong FH, Themmen AP, Fauser BC. Changes in anti-mullerian hormone serum concentrations over time suggest delayed ovarian ageing in normogonadotrophic anovulatory infertility. Hum Reprod 2004;19:2036–42.
- Davison SL, Bell R, Donath S,Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab 2005;90:3847–53.
- Alsamarai S, Adams JM, Murphy MK, Post MD, Hayden DL, Hall JE, et al. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab 2009;94:4961–70.
- Hudecova M, Holte J, Olovsson M, Sundstrom Poromea I. Long-term follow-up of patients with polycystic ovary syndrome: reproductive outcome and ovarian reserve. Hum Reprod 2009;24:1176–83.
- Tehrani FR, Solaymani-Dodaran M, Hedayati M, Azizi F. Is polycystic ovary syndrome an exception for reproductive aging? Hum Reprod 2010;25:1775–81.
Supplemental Material
POLYCYSTIC OVARY SYNDROME IN ADOLESCENCE
There is no agreement concerning how to diagnose polycystic ovary syndrome (PCOS) in adolescence. In fact, during the transition of girls into adulthood, several features may be in evolution or may only be transitory findings (1). Prematurely assigning a diagnostic label of PCOS to an adolescent may be incorrect and may result in unnecessary treatments and worsen the psychological distress that, especially in adolescence, is associated with disorders and therapies involving body image and reproductive issues. It may also jeopardize clinical and basic studies of PCOS, which require homogeneity and certainty about the diagnosis. Because of such problems, some investigators have even suggested avoiding making the diagnosis until the age of 18 years (2), and others have proposed specific and very strict criteria for the diagnosis (3). However, in girls who are affected by PCOS, it may be useful to start treatment during the adolescent years. Hence, by critically reviewing elements that may emerge during adolescence and are thought to encompass the diagnosis of PCOS, we suggest possible criteria upon which to base the diagnosis.Hyperandrogenism
In adult women, the diagnostic criteria include hirsutism, acne, or androgenic alopecia as markers of hyperandrogenism. However, acne is quite common during the adolescent years, and in most patients it is a transitory phenomenon (4). In addition, very few data exist about adolescent androgenic alopecia. Accordingly, we do not support the use of these clinical markers as criteria for the diagnosis of PCOS in adolescents.Hirsutism may represent a better marker of hyperandrogenism, and some investigators have reported that progressive hirsutism during the adolescent years may be an important sign of PCOS (5). However, in adolescents, it may be more prudent to rely on the presence of hyperandrogenemia, a classic characteristic of PCOS that generally appears at puberty, rather than on clinical signs for the diagnosis of PCOS. In fact, hyperandrogenemia is relatively constant and may represent an important symptom of PCOS during adolescence (6).
Chronic Anovulation and Menstrual Irregularities
\Chronic anovulation, generally presenting with oligomenorrhea or secondary amenorrhea, is one of the key elements for the diagnosis of PCOS in adults. However, chronic anovulation and menstrual irregularities are very common in adolescence, and approximately 40% to 50% of adolescent girls have anovulatory cycles (7, 8). There is a progression toward more ovulatory cycles with increasing gynecologic age; the prevalence of ovulatory cycles increases from 23% to 35% during the first year after menarche to 63% to 65% in the fifth year after menarche (7, 8).Most adolescent girls become ovulatory with age, but half of adolescent girls who have oligomenorrhea or secondary amenorrhea are affected by a permanent ovulatory disorder (9). It would be important to distinguish these girls from those who will progress eventually toward ovulatory cycles. In a study that assessed endocrine and ovarian morphologic parameters in girls at the age of 15 years and again at age 18 (10), the investigators showed that persistent oligomenorrhea was not predicted by increased serum luteinizing hormone (LH), increased androgens, or polycystic ovaries on ultrasound. Persistent oligomenorrhea was present in 43% of girls who had normal androgen levels, in 40% of girls who had normal LH levels, and in 44% of girls who had normalappearing ovaries on ultrasound. Similarly, insulin resistance and body mass index (BMI) did not predict persistent oligomenorrhea; however, increased BMI was the major risk factor for the persistence of anovulation. The association of oligomenorrhea with increased androgens or polycystic ovaries may help predict the persistence of oligomenorrhea. The additional findings of elevated androgen testosterone (T) levels and polycystic ovaries but not increased serum LH levels were predictive of the persistence of oligomenorrhea at age 18.
Ovarian Findings on Ultrasound
In adolescents, the ultrasound examination is often performed abdominally rather than vaginally, even though the resolution of the scan is better vaginally. This problem is further magnified by the evolution of ovarian findings with age. Some girls will be found not to have polycystic ovaries but to have multifollicular ovaries as a stage of development. Using strict criteria for multifollicular and polycystic ovaries, the two entities should not be confused. However, because of the reduced resolution of abdominal scans, misinterpretation is possible. In a study of 73 adolescents with menstrual irregularities, polycystic ovaries were found in 41% and normal ovaries in 36% of the girls, and 23% had multifollicular ovaries (11). In adolescents with normal menses, a high prevalence of characteristic polycystic ovaries were found in otherwise asymptomatic girls (12), suggesting that the occurrence of this finding is high in the general adolescent population. It was also suggested that maximal ovarian size is expected to occur 1.25 to 3.8 years after menarche (13), which is an important consideration when placing an upper limit for ovarian volume for the diagnosis of PCOS in adolescents.Suggestions for the Diagnosis of PCOS in Adolescents
Based on few available data, guidelines for diagnosing PCOS during adolescence have recently been proposed (1). During adolescence, a positive diagnosis of PCOS should require all elements of the Rotterdam consensus (and not just two out of three). In addition, it may be better to define hyperandrogenism as hyperandrogenemia (elevated blood androgens found using sensitive assays) and discount clinical findings such as acne and alopecia, with the exception of documented progressive hirsutism. Oligo-amenorrhea should be present for at least 2 years, and the diagnosis of polycystic ovaries by abdominal ultrasound should also include increased ovarian volume (>10 cm³ ). Thus, the diagnosis of PCOS should be considered only in girls who had menarche at least 2 years before diagnosis. By use of these parameters, clinicians may confirm the diagnosis PCOS only in adolescents who have hyperandrogenism, oligo-amenorrhea, and polycystic ovaries on ultrasound. When the diagnosis cannot be confirmed, the patients should be observed closely until adulthood, and the diagnosis should be reconsidered if the symptoms persist.Management of Adolescent PCOS
In patients in whom the diagnosis of PCOS has been established, the main clinical problem is the control of menstrual cycles and the treatment of hirsutism. Generally, combined steroid oral contraceptive pills (OCPs)—either containing or not containing an antiandrogen—may be safely used, but the usual contraindications to administration should be carefully considered. Lipid patterns should be evaluated before and after a few months of treatment.Special attention should be given to body weight because it has been suggested that obesity during adolescence may be an important factor that conditions the evolution of ovarian function (14). Psychological and dietary treatment should be used in obese adolescent girls; in some patients, especially if glucose intolerance is present, metformin may be added. Many of the patients with milder phenotypes may only need symptomatic treatment of their hirsutism. It has been shown that cardiovascular and metabolic risks are much lower in patients with mild PCOS phenotypes. More prospective studies are needed and will be useful in understanding not only the correct criteria for making the diagnosis of PCOS, but also whether any treatment may be useful to prevent or reverse the evolution toward PCOS in adulthood.
HIRSUTISM/ACNE/ALOPECIA
Hirsutism
In women, hirsutism is usually manifested by excessive facial and/or body hair. There are three hair types. Lanugo hair grows on fetuses and is lost early after birth. Vellus hair occurs in childhood and appears as nonpigmented, soft, and short. Terminal hair is pigmented, coarse, and excessive in hirsute women. Androgens (T and dihydrotestosterone [DHT]) transform vellus to terminal hairs in androgensensitive areas.Various factors may influence hair growth, including ethnic differences, systemic factors, sex steroid production, and target organ responsiveness. For instance, Asians have less dense hair compared with Caucasians.In addition, hirsutism occurs in 60% to 80% of American women with PCOS but in only 20% of Japanese patients. It is unclear whether these differences are genetically determined. Systemic conditions such as hypothyroidism and growth hormone therapy may also be associated with hirsutism (15, 16). In addition, seasonal conditions may also affect hair growth, with higher rates observed during the summer as compared with the winter months (17).
In women with PCOS, hirsutism may result from the combined influence of increased androgen production, increased circulating free T, or greater androgen activity within the pilosebaceous unit. In general, the primary source of androgen overproduction is the ovary, although adrenal androgens are increased in about 30% of cases. Not uncommonly, this increased androgen output is enhanced by elevated levels of serum free T due to decreased sex hormone–binding globulin (SHBG) levels, particularly in obese women. Finally, local factors in skin may amplify the androgen effect through increased 5a-reductase and androgen receptor activity.
The etiology of approximately 70% of hirsutism in women is PCOS, but other diagnoses should be excluded. The list of differential diagnoses for hirsute women includes hyperthecosis, nonclassic adrenal hyperplasia, Cushing syndrome, thyroid dysfunction, and ovarian and adrenal androgen-secreting tumors. Androgen-secreting tumors are rare, with a prevalence of less than 0.5% in hirsute women. Although individuals with acromegaly and hyperprolactinemia have been reported to exhibit an increased prevalence of hirsutism, the likelihood of hirsutism as the initial presentation for these disorders is also exceedingly rare.
Acne
Acne is the most common skin disorder and affects approximately 40 to 50 million people in the United States (18). This condition results from the formation of comedones, due to sebum accumulation along with desquamated follicular epithelial cells, which allows colonization by the bacterium Propionibacterium acnes (19). Inflammatory propagation of comedones may lead to development of papules, pustules, and nodules. Androgens may worsen acne formation by increasing sebum production within the pilosebaceous unit. Most women with PCOS who have acne exhibit facial lesions, and up to 50% of individuals demonstrate lesions on the neck, chest, and upper back (20).Past studies have shown that androgen levels are elevated in women with acne, although the severity of acne has not been positively correlated with any particular hormone with the exception of the adrenal androgen dehydroepiandrosterone sulfate (21). Notably, several studies have demonstrated an inverse relationship with SHBG.
About 50% of normal women with acne do not have clinical or biochemical evidence of hyperandrogenism. Conversely, in many women with PCOS hirsutism is not associated with acne. These discrepancies may be due to local androgen bioactivity. It has been postulated that [1] androgen levels within skin are more important mediators of acne than circulating levels and [2] androgen receptors may exhibit variable sensitivity to androgens.
Alopecia
Alopecia is loss of terminal hair from some or all areas of the body, but usually refers to the scalp. The condition is a common cause of baldness in women and tends to progress slowly. The pattern of hair loss involves the crown, sparing the fontal hairline, or balding with bitemporal recession (22, 23). An early sign of alopecia may be a widened hair parting.Androgenic alopecia is generally a poor marker for hyperandrogenemia unless present in women with oligomenorrhea (24). However, it has been demonstrated that polycystic ovaries are more likely in women with alopecia than in those with normal hair growth (25). The mechanism for androgenic alopecia is not well understood. It has been shown that aromatase activity is decreased and androgen receptors overexpressed in balding versus nonbalding scalps (26). It is interesting that there is evidence of increased 5α-reductase and 17β-hydroxysteroid dehydrogenase (17β-HSD) activity in the area of the occiput, which is spared hair loss in alopecia (26).
Pilosebaceous Unit
The pilosebaceous unit is the target of androgen stimulation, and responsiveness is influenced by activities of local enzymes and the androgen receptor. The hair follicle is composed of an outer root sheath, a dermal papilla, and a sebaceous gland. Androgen receptors are primarily located in the dermal papilla with some expression in the outer root sheath. Conversion of testosterone to DHT is determined by 5a-reductase, of which there are two isoforms, type 1 and type 2. Type 1 is found in sebaceous glands and pubic skin, and type 2 is located primarily in the hair follicle (27). Both types exist in the outer root sheath with less expression in the dermal papilla. In addition, type 1 is markedly expressed in the distal portion of the sebaceous gland, and type 2 is located primarily in the proximal duct of the sebaceous gland. Increased activity of one isoenzyme compared with the other could contribute to the variable clinical picture seen in the hyperandrogenic woman when the degree of hirsutism is not compatible with the severity of the acne.Treatment of Hirsutism and Acne
For women with PCOS in whom hirsutism is a major concern, treatment is focused on reduction of androgen production, decreasing the fraction of circulating free T, and limiting androgen bioactivity at the hair follicle. In those women with PCOS with acne vulgaris, clinical benefit may be derived from any of these therapeutic modalities. Because medical treatment of hirsutism prevents or slows the formation of new terminal hair growth and does not impact existing hair follicles, the hirsute patient must allow terminal hairs to be shed before anticipating significant improvement. In general, 6 months of treatment is considered the minimal interval to detect differences in hirsutism.In women with PCOS, the main therapeutic emphasis has been inhibition of ovarian steroid production with the use of estrogen and progestins as in OCPs. In addition, spironolactone, an antiandrogen, has been used alone or in combination with OCPs to block androgen action at the hair follicles. Flutamide, an androgen receptor antagonist, and finasteride, a 5α-reductase type 2 inhibitor, are also antiandrogens. Insulin-sensitizing agents, such as metformin and pioglitazone, have minimal effectiveness on hirsutism and acne. Various combinations of medications such as OCPs with antiandrogens have been advocated to lessen prescribed doses and side-effects as well as to enhance the cosmetic outcome. In severe cases of acne in women with PCOS, isotretinoin can be of great benefit, although individual responses may not be uniform. However, isotretinoin is not effective for hirsutism and, in some cases, may result in alopecia. As a topical treatment, eflornithine hydrochloride has been effective in decreasing of unwanted facial hair. Eflornithine is an irreversible inhibitor of ornithine decarboxylase, an enzyme essential for synthesis of polyamines that are necessary for cell division. Depilatory agents, the majority of which contain thioglycolates, act to sever chemical bonds and dissolve the hair shaft. Side effects of these chemical compounds are similar to eflornithine.
MENSTRUAL IRREGULARITY
The clinical expression of PCOS varies but commonly includes oligo-ovulation or anovulation, hyperandrogenism (either clinical or biochemical), and the presence of polycystic ovaries (28, 29). Obesity and insulin resistance are commonly encountered (30) but are not required for diagnosis.Amenorrhea is generally defined when there are intervals between periods exceeding 199 days, whereas in oligomenorrheic patients the intercycle interval varies between 35 and 199 days. However, oligomenorrheic and, to a lesser extent, amenorrheic women with PCOS occasionally experience spontaneous ovulations. Indeed, the original manuscript of Stein and Leventhal mentioned the presence of ovulation stigmata on the enlarged polycystic ovaries of some patients. Information about spontaneous ovulation rates in oligomenorrheic and amenorrheic women is scarce (31). In the placebo arm of a large randomized controlled trial (RCT), women with PCOS ovulated in 32% of cycles, though this likely overestimates the overall rate (32) In a woman who has oligomenorrhea/amenorrhea, there is an approximately 90% chance that PCOS also will be diagnosed (33).
Menstrual Cycle Throughout Life
During the assessment of menstrual cycle length, one should bear in mind that oligo-ovulation and anovulation are quite common in adolescents. Menstrual cycles are rather long and very irregular during the first year after menarche. The establishment of regular menstrual cycles can be a slow process. It has been shown that 85% of menstrual cycles are anovulatory during the first year after menarche, and up to 59% of cycles are still anovulatory during the third year after menarche (34). This is especially important because PCOS is increasingly recognized as a major endocrine problem in adolescents (35).Moreover, it has been recognized that, due to aging, women with PCOS appear to gain more regular cycles as they approach menopause (31, 36, 37). Apart from age, the smaller follicle count, the higher follicle-stimulating hormone (FSH) serum levels, and the lower FSH-induced inhibin B increment found in aging PCOS patients confirm that this phenomenon is indeed due to ovarian aging (38). Finally, in line with the high levels of antimullerian hormone (AMH) in PCOS patients (39), their steady decline over time (40), and the strong association of AMH levels with the number of antral follicles (41), cycle irregularity improves with increasing age (38). It has been hypothesized that women with PCOS may have a longer reproductive life span (39, 42).
Incidence of Menstrual Cycle Irregularities in PCOS
The incidence of cycle irregularity in adolescent girls with PCOS seems to be quite variable. About 43% presented with oligomenorrhea, and 28% had either primary (7%) or secondary (21%) amenorrhea. Approximately 21% reported regular menstrual cycles, and 7% reported frequent menstrual cycles with a cycle interval of less than 21 days (35). Adult patients with PCOS present with either oligomenorrhea or amenorrhea in up to 95% of cases (33). Oligomenorrhea and amenorrhea have been encountered in women ranging from 45% up to 95%, depending on the definitions used to diagnose PCOS (43). If the U.S. National Institutes of Health (NIH) criteria are used, 100% of all women with PCOS will have irregular menstrual cycles; if the Rotterdam consensus definition is used, up to 30% will have regular menstrual cycles(30, 44, 45).Menstrual Cycle Irregularities and Other PCOS
Characteristics Several studies had assessed the endocrine profile in patients with PCOS with either regular cycles or with oligomenorrhea or amenorrhea. All studies point in a similar direction indicating a more deranged endocrine profile in those women with irregular cycles compared with those with regular cycles. Moreover, women with amenorrhea and PCOS constitute the group with the most profound derangements: they are significantly more hyperandrogenic, have increased serum luteinizing hormone (LH) and cortisol levels, and are more hyperinsulinemic (46, 47). In an analysis of a large data set of nearly 2,000 women with PCOS, amenorrheic women were shown to be the most profoundly hyperandrogenic, and they had statistically significantly higher T, androstenedione, dehydroepiandrosterone (DHEA), and dehydroepiandrosterone sulfate (DHEAS) levels with lower SHBG serum concentrations compared with woman with oligomenorrhea. Amenorrheic women also had the highest antimullerian hormone (AMH) serum concentrations and higher antral follicle counts compared with both oligomenorrheic women and regularly cycling patients with PCOS (31). In a recent study, the number of follicles seemed to be similar between women with PCOS having irregular or regular menstrual cycles (48). It therefore seems that women with amenorrhea and PCOS constitute the most profoundly affected phenotype. Indeed, the incidence of ovulatory cycles was statistically significantly lower in women with amenorrhea compared with those with oligomenorrhea (49). Similarly, a higher socioeconomic status was associated with a higher prevalence of the ovulatory phenotype. Differences in ovulatory status were also attributable to differences in serum insulin levels and fat quantity and distribution (50).Menstrual Cycle Irregularities and Fertility/Fecundity
Women with self-reported oligomenorrhea or amenorrhea and/or hirsutism had similar pregnancy rates and abortion rates compared with the general population without these symptoms. However, symptomatic women more often experienced infertility. Oligomenorrhea or amenorrhea as well as obesity were more often associated with decreased fecundability (51). Similarly, amenorrhea and oligomenorrhea also predict the outcome of first-line ovulation-induction treatment. Along with BMI, mean ovarian volume, and the free androgen index, the degree of menstrual cycle irregularity predicted ovulation in women with anovulatory infertility (52). Moreover, age along with amenorrhea predicted livebirth rates in these women (52). During second-line treatment, the menstrual cycle pattern is one of the most significant predictors of live birth (53). Significantly fewer women with amenorrhea ovulated on clomiphene citrate compared with those with oligomenorrhea. During second-line treatment, women with oligomenorrhea had a reduced chance of conceiving and had reduced live-birth rates when compared with those with amenorrhea (49).The Menstrual Cycle and Quality of Life
There are several studies addressing psychological well being in women with PCOS. They all indicate that the symptoms typically associated with PCOS such as acne, hirsutism, irregular menses, amenorrhea, obesity, and subfertility are a major source of psychological morbidity and can negatively affect quality of life (54). In PCOS, changes in appearance (particularly obesity and hirsutism) reduce quality-of-life and decrease sexual satisfaction. The roles of biochemical, endocrine, and metabolic parameters as well as menstrual irregularities appear to be less important (55). Amenorrhea in women with PCOS was associated with lower self-esteem, greater fear of negative appearance, and earlier sexarche compared with a nationwide Dutch control cohort. These results suggest that in younger women sexarche was earlier compared with a historic Dutch control cohort whereas in older patients with PCOS sexarche seemed to be delayed (56).The Menstrual Cycle and Long-Term Health Risks
Although clinical parameters such as ovulation disorders and hirsutism play a key role in the definition, there is a paucity of data that correlate the grade of clinical findings with metabolic disorders in PCOS. This is surprising because, for example, the degree of cycle irregularity as a rough parameter of oligo-ovulation/anovulation is an objective and reproducible parameter that can be easily assessed (47). Indeed, several studies indicate that oligomenorrheic or amenorrheic women exhibit a significantly more pronounced degree of insulin resistance, are more obese, and have more disturbed carbohydrate metabolism.Moreover, amenorrheic patients have a less favorable metabolic profile (47). Irregular menstrual cycles along with hirsutism particularly seem to constitute an unfavorable combination as far as the metabolic syndrome is concerned, and both factors might be helpful in identifying women with metabolic and cardiovascular risk factors associated with PCOS (57, 58). Moreover, the observed changes in endocrine and metabolic profiles, also recorded in obese and overweight controls, were magnified in the presence of symptoms associated with PCOS like oligomenorrhea or amenorrhea and hirsutism (46). In a large Dutch cohort of patients with PCOS, it was established that obesity, rather than the menstrual cycle pattern or the size of the follicle cohort, determined hyperinsulinemia, dyslipidemia and hypertension in aging women with PCOS (59).
The relationship between endometrial cancer risk due to prolonged unopposed estrogen exposure and PCOS has been established for several decades (60). The intermenstrual interval seems to correlate well with endometrial thickness and endometrial hyperplasia in women with PCOS (61). Compared with a control group, proliferative endometrium was significantly more often observed in patients with PCOS compared with age-matched controls.
CONTRACEPTION
Combined oral contraceptive pills (OCPs) have been the mainstay for the chronic treatment of women with PCOS not seeking pregnancy (62). Oral contraceptives can address many of the goals of reproductive-aged women with PCOS: they ameliorate hyperandrogenic skin manifestations, regulate menstrual cycles, and thereby lower the risk of endometrial carcinoma and provide effective and safe contraception.Oral contraceptives suppress the secretion of LH and lead to a decrease in ovarian androgen production. The estrogenic fraction increases the levels of SHBG, which, in turn, results in a decrease in free T levels. The progestin in the pill can compete for 5a-reductase and the androgen receptor, and thus antagonize androgen action. Oral contraceptives also decrease adrenal androgen production by a mechanism that is as yet unclear, possibly due to a decrease in adrenocorticotropic hormone (ACTH) levels.
Almost all of the OCPs contain ethinyl estradiol as the estrogenic fraction, but progestins in the pills vary in their androgenic potential. Norethindrone, norgestrel, and levonorgestrel are known to have androgenic activity, whereas desogestrel, norgestimate, and gestodene are less androgenic. The pills containing progestins with antiandrogenic activity, rather than second- and third-generation OCPs containing progestins with varying androgenic activity, appear to be an appropriate alternative in the treatment of PCOS. Among OCPs containing antiandrogenic progestins, the combination of ethinyl estradiol and cyproterone acetate has been used in many studies of PCOS, whereas only a few studies of ethinyl estradiol in combination with drospirenone or chlormadinone acetate are available, and the combination of ethinyl estradiol and dienogest has not yet been studied in PCOS.
Taken together, OCPs are effective agents for the longterm management of PCOS. However, there are concerns about the safety of these drugs, particularly potential longterm risks related to cardiovascular disease (CVD) and glucose intolerance.
OCP Use and Cardiovascular Disease Risk in PCOS
Current use of low-dose OCPs in healthy women might increase CVD risk although that risk does not continue once OCPs are stopped (63, 64). The CVD risk is associated with increased age, smoking, and hypertension (63). Accordingly, young nonsmoking women do not appear to possess any increased CVD risk due to OCP use. Intriguing data from a healthy population suggest that OCP use during the reproductive years might be protective against CVD later in life (64). Unfortunately, there are no data available in the literature assessing the potential association of OCP use and CVD outcome in PCOS. Because patients with PCOS do not have increased or premature morbidity or mortality from CVD despite having increased CVD risk factors, it would be interesting to test the hypothesis that long-term use of OCPs during reproductive years might be protective against CVD morbidity and mortality in PCOS later in life.OCP Use and Diabetes Risk in PCOS
The strong association between PCOS and insulin resistance and the increased risk of diabetes in this disorder have boosted the number of insulin-sensitizer studies in PCOS within the last 15 years. On the other hand, there have been only a few studies evaluating the metabolic effects of OCPs in PCOS. Similarly, there are few randomized double-blind studies are available in the literature comparing the metabolic effects of an OCP either with another OCP or with an insulin sensitizer or with an OCP/insulin-sensitizer combination (65).A recent Cochrane review comparing OCPs versus metformin in PCOS identified only four RCTs in the literature involving 104 patients with a study duration ranging between 6 and 12 months. This review reported, based on limited evidence, that OCP use does not have any adverse metabolic risk compared with metformin use (66).
There are a few prospective studies on a limited number of patients using different combinations of OCPs with a duration ranging from 3 to12 months (65). The results regarding the effects of OCPs on insulin sensitivity, measured by various methods ranging from fasting insulin to clamp studies, are inconsistent and contradictory in that decreased, unchanged, and increased insulin sensitivity measurements have been reported (65). More importantly, in all but two studies glucose tolerance status did not change. Of note, these two small studies included morbidly obese patients with PCOS with an average BMI of 36.8 and 37.2 kg/m² , respectively. Additionally, potential confounding factors that might have influenced the results, such as ethnicity, family history of diabetes, and level of physical activity, were not reported (67, 68).
Overall, limited available data suggest that low-dose OCP use for up to 1 year does not have an adverse impact on insulin sensitivity in most patients with PCOS even though decreased or increased insulin sensitivity might be observed in a small group of individuals. Low-dose OCP use for 1 year or less does not appear to increase the incidence of type 2 diabetes in PCOS. However, deterioration of glucose tolerance status might be observed, particularly in morbidly obese patients with PCOS. It is highly likely that, similar to healthy individuals, the risk of diabetes development depends on individual patient characteristics such as BMI, age, ethnicity, and family history of diabetes as well as on individual characteristics of the OCP combination. Finally, it remains to be determined prospectively whether these variable effects of OCPs on insulin sensitivity and glucose tolerance status for less than a year persist with longer term use of these medications in PCOS.
Long-Term Use of OCPs and Cardiometabolic Risk in PCOS
Only a few long-term observational studies evaluating the metabolic effects of OCPs in PCOS are available in the literature. In a prospective open-label study evaluating lipid profiles and glucose homeostasis in 72 women with PCOS treated with ethinyl estradiol/cyproterone acetate for 3 years, the investigators observed an increase in triglycerides and high-density lipoprotein (HDL) cholesterol and a decrease in the low-density lipoprotein (LDL)/HDL ratio in women PCOS after the treatment. More importantly, insulin and glucose plasma concentrations did not change (69).Another small observational study on 37 patients with PCOS with an average follow-up period of 10 years (range: 12 to 180 months) assessed the long-term effects of OCPs on cardiometabolic risk factors in patients with PCOS (70). Sixteen patients were on OCP treatment while 21 patients had never used OCP. None of the anthropometric measurements, including body weight, BMI, waist and hip circumference, and waist-to-hip ratio (WHR), changed in non-OCP users during the follow-up period. Alternatively, waist circumference and WHR statistically significantly decreased in OCP users. The area under the curve (AUC) for glucose during oral glucose tolerance test (OGTT) administration decreased in OCP users and remained unchanged in non-OCP users whereas the AUC for insulin remained unchanged in OCP users but increased in non-OCP users. Finally, HDL-cholesterol and SHBG levels increased significantly only in the OCP users while there was no change in non-OCP users (70). Taken together, findings of these studies suggest that insulin resistance worsens during the natural course of the syndrome while long-term OCP use either does not change or improves the cardiometabolic risk parameters, including insulin resistance, lipoprotein profile, and potentially body fat distribution.
Current Contraindications to the Use of OCPs
The World Health Organization (WHO) has developed an evidence-based guideline for the use of OCPs (71). This document, based on systematic reviews of available clinical and epidemiologic research, is updated regularly. The most recent version was published in 2009. Absolute contraindications to the use of low-dose combined OCPs include smoking (more than 15 cigarettes a day) over the age of 35, liver dysfunction, hypertension (>160/100 mm Hg), deep venous thrombosis, ischemic heart disease, stroke, and breast cancer.Conclusions
Oral contraceptive pills are a key component in the chronic treatment of PCOS, addressing many of the goals of reproductive-aged women with PCOS who are not seeking pregnancy. Only a few studies assessing the metabolic effects of OCPs in PCOS are available in the literature. There are even fewer RCTs. Most of the studies included only a small number of participants with a limited follow-up period, and several confounding factors that might have influenced the results were not taken into account in these studies. Long-term risks and benefits of the use of other modalities remain largely unknown, including [1] contraceptives delivered by nonoral routes (i.e., transdermal or vaginal) (72, 73), [2] OCPs combined with other treatment modalities such as antiandrogens or insulin sensitizers, and [3] progestin-only contraceptives.Larger randomized, controlled studies are needed to resolve controversies about OCPs and to address important questions raised by earlier studies in the literature. New low-dose OCPs containing antiandrogenic progestins with a better safety profile are now available. Accordingly, future studies evaluating the long-term effects of OCPs in the treatment of PCOS should adequately consider clinical heterogeneity of the syndrome and variations in the efficacy and safety of different combinations.
Despite the unresolved questions about overall long-term safety and potential variable effects in different phenotypes, current evidence supports the benefits of long-term OCP use in PCOS. However, it should be kept in mind that OCP use might increase the risk of diabetes, particularly in obese patients with severe insulin resistance. Continued development of effective long-term treatment options for PCOS is needed. Meanwhile, it is critical that the WHO guidelines for the contraindications to OCP use should be observed in women with PCOS and that the precise individualized treatment targets and risk stratification depending on patient characteristics be determined.
QUALITY OF LIFE
The present review updates studies published since the PCOS reviews performed on quality of life (54), specific psychological impairments (74), and psychological and lifestyle interventions (75–78) were reported.PCOS Effects on Quality of Life (QOL)
Seven new quality of life (QOL) studies have been published, and the conclusions remained more or less the same as those published earlier. In-depth interviews indicate that women with PCOS feel different, especially in regard to their femininity (79). Women use words such as ‘‘freakish’’ and ‘‘abnormal’’ to describe their periods, ‘‘upsetting’’ and ‘‘embarrassing’’ to describe excess body hair, and ‘‘crushing’’ to describe fertility problems (80). In generic instruments, patients with PCOS report lower QOL (Short Form 36 Health Survey [SF-36]: physical, psychological) compared with community controls (81), healthy controls matched for body mass index (BMI) (82), and population reference groups (83). In online studies, women with self-reported PCOS reported more unfavorable PCOSQ and generic SF-36 scores on all dimensions compared with respondents not declaring PCOS (84, 85).More than 20 studies investigating specific impairments in patients with PCOS have been added to the literature since a 2006 review on specific disorders (74). Across these studies, which used questionnaires (86), structured psychiatric interviews (87), and Internet methods (84, 85, 88–90), there is convergence that the prevalence of emotional disorders (depression, anxiety) is higher in patients with PCOS compared with healthy controls (85, 91–93) and compared with normative data from age-matched reference groups (83, 93). Studies have replicated the greater prevalence of emotional disorders in adolescents (i.e., 12 to 18 years) (94– 96) and younger women (i.e., 18 to 25 years) (88) compared with controls. Higher prevalence rates were also reported in samples from Turkey (97) and India (98), countries not represented in the earlier review. One Italian study reported the rate of depression in new cases (no previous diagnosis, no previous treatment) of PCOS (88% participation rate) versus agematched controls (mean age: 17 years) (95). Patients with PCOS were more likely to have mild depression (27%) compared with controls (9%) with no difference in more severe forms of depression. The only longitudinal investigation comparing rates of major depressive disorder at two time points in the same sample showed that an additional 19% of patients with PCOS developed major depressive disorder in the 22- month period after medical assessment (87, 99).
Four studies examined other dysfunctions. A case-control study did not find a statistically significant difference in the rate of sexual dysfunction between Italian patients with PCOS and healthy, age- and BMI-matched controls (4% and 11%, respectively) (100). In contrast, for 64% of patients in a descriptive American study, the women with PCOS scored above norms on the sexual distress inventory (101); in a Polish study, the patients with PCOS reported statistically significantly more sexual dissatisfaction (28%) than the controls (10%) who were presenting for other obstetric/gynecologic complaints (81). In a recent study, patients with PCOS were found to have started sexual intercourse (i.e., sexarche) at an earlier age than the reference population (56). An earlier case-control study with American adolescent patients with PCOS (mean age: 17 years) versus healthy controls showed no difference in the timing of sexarche (102). Statistically significantly more patients with PCOS compared with community controls had an eating disorder (12% vs. 4%) and/or social phobia (27% vs. 2%) based on structured clinical interviews (91).
PCOS Correlates Associated with Poor Quality of Life
Relationships between the features of PCOS (e.g., obesity) and QOL have been investigated to identify which feature is most problematic and causes specific consequences (e.g., emotional disorders). Several studies report an association between weight or BMI and QOL scores (83, 84) and recurrent major depressive disorder and social phobia (91). In an Australian study, the difference between PCOS and community controls in emotional disorder was attenuated after controlling for BMI (88–90). In two intervention studies, the extent of weight loss was associated with improvement in QOL (94, 103). In a Polish sample with comparatively lower BMI scores, QOL was not associated with BMI (81). Experimental work suggests that patients with PCOS may have impaired immune function (most likely due to obesity), and that immune dysregulation is associated with mood (82).Other determinants of QOL seem to produce more variable effects. For example, one study reported that QOL was associated with an unfulfilled wish for a child (85, 86), but another did not find this to be the case (83). Other determinants are too sparsely studied to draw firm conclusions. An association was reported between PCOS status and sleep disturbance, anxiety disorders (phobia), and pain (92). Self-reported hirsutism, weight issues, and infertility were all associated with QOL measures (generic questionnaire and PCOSQ) in an Australian study (93).
Impact of Interventions on Quality of Life
Weight loss has been the main target of interventions. An RCT was conducted of metformin versus placebo and incorporated a 24-week lifestyle modification program (LMP) for adolescents with PCOS and their parents. All PCOSQ domains of QOL improved from baseline to immediately after intervention (94). Effects of a 24-week LMP focused on changing physical activity level or diet (depending on patient preference) in obese women with PCOS were also studied. There was no difference between the activity and diet groups on frequency of sexual activity (only QOL outcome), but physical and biochemical outcomes improved after both interventions (104). Women with PCOS randomized to diet only, diet and aerobic exercise, or diet and combined aerobic-resistance exercise showed significant decreases in depression scores by week 10, with no further change by the end of the program (week 20) (103). Improvements in PCOSQ subscales of emotion, body weight, and menstrual problems were also clinically significant. Finally, an investigation of laser surgery showed significantly improved QOL scores after five treatments (laser hair removal) (105). Participants of a nurse-led support group (providing emotional expression and support) for patients with PCOS were interviewed, and qualitative analysis reported the benefits in increased knowledge, reduced isolation, and enabling change (106).Methodological and Conceptual Considerations
There is consistent evidence that patients with PCOS represent an at-risk group for a variety of psychological and behavioral disorders and for reduced QOL. Because of the complicating methodologic and conceptual issues, it is difficult to deduce from existing research what proportion of patients with PCOS fall into the at-risk group. Many studies providing prevalence estimates do not have the appropriate design for this type of inquiry. For example, recruitment from online sites or patient advocacy groups (86), advertisements appealing for particular subpopulations (e.g., ‘‘body weight concerns’’; and high attrition in intervention studies) (78) can contribute to biased prevalence values and discrepancies across studies. Other sources of bias are due to the diagnosis being selfreported (84, 107) or assessed by interviewers who were not blind to the study hypotheses (87).The nature of PCOS may also impact on prevalence. The multiple phenotypes and manifestations of PCOS across the life span (108) mean that developmental changes in the disorder may produce different psychosocial and symptom profiles over time. Additionally, because the onset of PCOS is rarely known, one cannot disentangle potential features of the disorder from reactions to it. Results suggest that there is psychological disadvantage even before diagnosis (95), but the comparatively higher prevalence reported in older women with PCOS suggests there may be strong reactive effects and/or aggravation of the disorder over time. Even if there was a reliable methodology, the cross-cultural and race variations in disease manifestation (e.g., hirsutism) and country variations in the correlates of PCOS may make cross-study synthesis difficult.
Attention should be given to the assessment tools because these too can yield different prevalence estimates. For example, subjective appraisal and medical evaluation yield different estimates of sexual dysfunction (100, 101). Patients with PCOS also respond differently to experimentally induced stressors (public speaking) compared with controls (i.e., amplified cardiovascular and hormonal response, attenuated immune response) despite similar subjective ratings of emotional distress (107). The only PCOS-specific QOL tool (PCOSQ) has important limitations. The Emotion subscale is intended to capture the impact of PCOS on mood and emotions, but the questions refer to specific PCOS features (‘‘weight concern,’’ ‘‘infertility concern’’), virtually guaranteeing that groups with PCOS appear to have more unfavorable emotional well-being scores and/or that BMI or infertility status is shown to account for unfavorable emotional well-being. Others have argued that PCOSQ is a measure of symptom issues rather than of QOL, and these limitations need to be addressed before this instrument can be used in clinical trials (109). Together, these issues imply that the confidence intervals for the prevalence estimates and/or effect size for PCOS effects are undoubtedly wide.
Conclusions
Further efforts to determine the true prevalence of emotional and behavioral disorders (and reduced QOL) in PCOS should only be made if designs capable of achieving this goal are employed.The existing research does not yet provide compelling support for prior recommendations of psychological screening for all patients with PCOS to improve long-term prognosis (74). Screening is most appropriate when the screened-for problem (e.g., depression or sexual dysfunction) can be detected at the start of treatment with relatively low cost, when there are effective and acceptable methods to detect the problem and the consequences of the disorder are known and significant, and, importantly, effective interventions to tackle the problem exist (110). Without a true prevalence estimate for psychological disorders/low QOL in PCOS (5%? 30%?), the cost of screening cannot be determined. Further, the management of depression or sexual disorders in populations of women with PCOS has not been systematically studied to the same extent as obesity, and thus there is uncertainty about what interventions to offer identified patients. Effective treatments exist for some disorders, but whether they would be acceptable and feasible to patients with PCOS is not known. High dropout rates for lifestyle management programs in obese patients with PCOS (78) and low counseling uptake in infertile patients (111) suggest that continued intervention development is required before rolling out screening programs.
PREGNANCY
Women with polycystic ovary syndrome (PCOS) are subfertile, with multiple potential contributing factors, including the effects of obesity and metabolic, inflammatory, and endocrine abnormalities on ovulatory function, oocyte quality, and endometrial receptivity and fetal development. Women with PCOS may exhibit reduced developmental competence of the oocyte, defined as the ability of the oocyte to complete meiosis, achieve fertilization, and develop into a normal embryo. Impaired oocyte competence in PCOS is inextricably linked with abnormal follicle development. Ovarian hyperandrogenism and hyperinsulinemia may promote premature granulosa cell luteinization, and paracrine dysregulation of growth factors may disrupt the intrafollicular environment, alter granulosa cell–oocyte interactions, and impair cytoplasmic and/or nuclear maturation of oocytes (112). However, these features are not universal, and oocyte quality, fertilization, and implantation rates in women with PCOS may be normal (113).Abnormal Folliculogenesis and Implantation
During follicle growth and development, the growing and maturing oocyte obtains nutrients and energy substrates from the reservoir provided by the follicular environment. Polycystic ovary syndrome is associated with metabolic disturbances, including impaired insulin signaling and glucose metabolism in ovarian follicles. Studies of granulosa cell function from in vitro fertilization (IVF) patients with PCOS have established that follicular glucose metabolism is altered, with cultured cells showing a significant reduction in insulinstimulated glucose consumption (114). It is likely that the metabolic lesion in the follicle precipitates an altered metabolic milieu throughout oogenesis, which may have downstream consequences for oocyte energy generation and/or could result in alteration of the metabolic profile of the oocyte enclosed within the follicle. Reduced expression of genes encoding oxidative phosphorylation components has been observed in women with PCOS (115). An additional factor associated with aberrations in oocyte meiosis in PCOS is altered expression of key genes associated with chromosome alignment and segregation, which has been attributed to hyperandrogenemia (116). Indeed, it has recently been shown that differences in metabolism exist in oocytes derived from PCOS patients (117). Oocytes from PCOS patients are not likely to harbor an increased number of numeric chromosome abnormalities, but the disturbed metabolic profile of oocytes is associated with chromosomal predivision (i.e., premature separation of sister chromatids) (117).During early pregnancy, the embryo may be exposed to androgen excess in utero, which may have long-term effects, particularly on female offspring. Fetal hyperandrogenism may disturb epigenetic programming, in particular those genes regulating reproduction and metabolism (118, 119). The risk of miscarriage in PCOS patients after natural or assisted conception is reported to be as high as 30% to 50%, and it has been suggested that the miscarriage rate is threefold higher in women with PCOS than in normal women (120). However, other data from prospective, randomized clinical trials suggest that the miscarriage rate is in the range of 15% to 25% and that it is comparable with other subfertile populations (121–123). The role of endometrial dysfunction in women with PCOS is under investigation (124).
Pregnancy Complications
When pregnancy occurs in women with PCOS, there is a higher incidence of gestational GDM (40% to 50%) (125), gestational hypertensive disorders (e.g., preeclampsia and pregnancy-induced hypertension) (5%), and the birth of small-for-gestational-age (SGA) babies (10% to 15%) (126). In addition, GDM may also result in fetal macrosomia. The potential mechanisms for these problems include obesity, altered glucose metabolism, and disturbances in uterine blood flow. Indeed, alterations in the impedance to blood flow through the uterine artery may be sustained during first and middle trimester of pregnancy (127). Obesity in its own right is associated with several adverse pregnancy outcomes, including spontaneous miscarriage, preeclampsia, gestational diabetes, congenital anomalies (e.g., cardiac and spina bifida), fetal macrosomia, cesarean delivery, and wound complications after cesarean section (128). Insulin resistance partially mediates the effects of obesity on adverse pregnancy outcome (129). However, studies on GDM prevalence in women with PCOS show conflicting results, reflecting the heterogeneity of PCOS and the diversity in methodology of screening and diagnosing GDM (126).In a meta-analysis in which pregnancy outcomes in women with PCOS were compared with controls, 15 studies were included, involving 720 women presenting with PCOS and 4,505 controls (126). Women with PCOS demonstrated a statistically significantly higher risk of developing GDM (odds ratio [OR] 2.94; 95% confidence interval [CI], 1.70– 5.08), pregnancy-induced hypertension (OR 3.67; 95% CI, 1.98–6.81), preeclampsia (OR 3.47; 95% CI, 1.95–6.17), and preterm birth (OR 1.75; 95% CI, 1.16–2.62). Their babies had a statistically significantly higher risk of admission to a neonatal intensive care unit (OR 2.31; 95% CI, 1.25–4.26) and a higher perinatal mortality (OR 3.07; 95% CI, 1.03–9.21), unrelated to multiple births (126).
Treatment with Metformin
The use of metformin for women with anovulatory PCOS was found to have no benefit with respect to enhancing either fertility or live-birth rates (76, 77, 122), and its routine use is not recommended. Similarly, a recent meta-analysis showed metformin had no effect on the miscarriage risk in PCOS patients when administered before pregnancy (130). One small RCT did, however, report a reduced rate of severe pregnancy complications when metformin was taken through pregnancy (131), although a subsequent larger multicenter trial by the same group found no benefit of metformin in reducing pregnancy complications or altering fetal weight (123).ETHNIC DIFFERENCES IN THE PHENOTYPE
A study of ethnic variations in the expression of PCOS requires evaluation of epidemiologic data based on the geographic location of affected women, with particular reference to its phenotype, comorbidities, and response to treatment. This requires a systematic review of population-based data or of large clinical databases to ascertain the racial and cultural determinants of manifestations of PCOS such as hyperandrogenism, but also obesity, insulin resistance, and metabolic risks, and hyperandrogenism. Standard continent-based categorization of human populations includes African, Caucasian (Europe and Middle East), Asian, Pacific Islander (Australian, New Guinean, and Melanesian), and Native American groups. However, this method pays little attention to differences within the groups living in the large continents—particularly in Asia. Because there are distinct differences between East Asians (e.g., Chinese and Japanese) and South Asians (e.g., Indian, Bangladeshi, Sri Lankan, and Pakistani) that have an impact on PCOS, a further subcategorization of Asians into East, South-East, and South Asians is required.Available data depict variations of the PCOS phenotype among Caribbean Hispanics (132), Mexican Americans (133–135), Japanese (136), indigenous Chinese/Taiwanese (137–140), migrant and indigenous South Asians (141–146), Thai (147, 148), multiethnic groups living in the United States (149–153), Southern Europeans (154–156), New Zealand and Canadian indigenous groups (157, 158), and migrant and indigenous Arabs (159–161). These add new dimensions to the ethnic variability in the phenotype of PCOS, its diagnostic criteria, metabolic problems, and correlates as well as the psychosocial aspects affecting quality of life (QOL) and health-seeking behaviors.
Ethnicity and Metabolic Phenotypes
Ethnic variation does occur in the metabolic phenotype of PCOS (obesity, acanthosis nigricans, and insulin resistance) and in the androgenic phenotype (pilosebaceous unit sensitivity to androgens, acne, and temporal balding). Asian women generally are shorter, have a lower BMI, and have a ‘‘milder’’ phenotype in terms of hyperandrogenism (149, 151), whereas South Asians in particular have a high prevalence of the metabolic syndrome and risk of type 2 diabetes (T2D), and their central obesity more than their BMI reflects their metabolic risk (144, 162). East Asians manifest hirsutism to a very mild degree compared with other ethnic groups (137, 163). However, their propensity to acne appears to be more marked than in South Asians (139, 140). The risk of metabolic disease is lower in East Asians than among South Asians (138). African American and Hispanic women are more obese and more prone to metabolic problems; women of African descent are particularly prone to hypertension and cardiovascular disease, whereas Hispanic women are more at risk for metabolic syndrome and T2D (149–153). Compared with South Asian women, women of Middle Eastern origin are more obese, and metabolic disease is a common problem (160, 161), although it is not as marked in terms of the hypertension and diabetes developed in young South Asians. There is a striking prevalence of hirsutism among Arab women, which is similar to that seen in women of Mediterranean origin (156). Nevertheless, abnormal glucose tolerance in southern and eastern Europeans (164) is far less common than in South Asians and Hispanics.Groups at risk for the metabolic syndrome and T2D (e.g., South Asians), may manifest PCOS at a younger age (late teens and mid 20s). A common clinical indicator of their greater metabolic risk is acanthosis nigricans, which is not, however, a diagnostic marker for PCOS (144, 148, 165). A family history of T2D correlates with the metabolic risks of PCOS, which are influenced by ethnicity (150). Moreover, hyperandrogenemia shows less correlation with metabolic problems in these high-risk ethnic groups. Meanwhile, white European women are taller in stature and have a moderate metabolic risk, which manifests later in life (fourth decade). It is interesting that white Europeans of the same origin but living in different locations have some differences in manifestation (e.g., Boston vs. Iceland) (152, 153). But migration is not a determinant of the development of metabolic problems among South Asians with PCOS.
Acknowledging the Full Impact of Ethnicity and Origin on the PCOS Phenotype
The health-seeking behavior of women with PCOS is culturally determined; for instance, South Asians are reclusive, with a culture of silence (33). The impact on QOL also appears to have an ethnic basis. For example, the QOL of affected South Asian women shows a greater impact from hirsutism than from obesity (166), which is mirrored by reports of migrant Arabs (159). Such misconceptions of obesity can lead to failure to recognize ethnic-specific problems in managing PCOS.Geographic location, ethnic origin, and cultural/social practices are likely contributors to the differing manifestations of PCOS and should be recognized in routine clinical practice. Ethnic groups from South Asia manifest PCOS at a young age, and they have a relatively larger waist circumference despite a smaller body configuration. They have a higher risk for the metabolic syndrome, which correlates with acanthosis nigricans and a family history of diabetes rather than with hyperandrogenism. African American women are at greater risk of obesity and hypertension than diabetes. Available data support the recommendation to adopt a policy of training primary health caregivers from resource-limited settings in developing countries to evaluate symptomatic young women for PCOS. This should be combined with simple methods of risk assessment for metabolic disease by measuring waist circumference and blood pressure and identifying acanthosis nigricans to institute appropriate preventive care.
OBESITY
It is known that PCOS is associated with obesity, but the prevalence of obesity in PCOS populations has not been systematically assessed. In a literature search on obesity and PCOS, there was widespread variability in the prevalence of overweight women (BMI 25 to <30 kg/m² ) in PCOS population across different countries. The proportion of women with PCOS who were overweight ranged from 10% in Italy (167) to 37% in Kuwait (168). The largest studies in this sample (n > 400) were conducted in Australia (93) and the United States (169). These studies reported that 15% to 19% of women with PCOS were overweight. The proportion of adolescents with PCOS who were overweight was between 18% and 22%, as noted in two studies conducted in the United States(170, 171).Prevalence of Obesity
The prevalence of obesity (BMI >30 kg/m² ) was greater than the prevalence of overweight (BMI 25 to 30 kg/m² ) in women with PCOS. As with the prevalence of being overweight, the prevalence of obesity varied widely across geographic regions. The lowest proportion of obese women with PCOS (25%) was noted in a study conducted in Pakistan (172). The highest prevalence of obesity was reported by studies conducted in the United States and Australia, with 61% to 76% of women with PCOS considered obese (93, 169).When the overweight or obese women with PCOS were combined in the consideration for prevalence, the proportion of women with a BMI greater or equal to 25 kg/m² ranged from 20% in China (8) to over 90% in Poland, the United States, and the United Kingdom (169, 173, 174). The larger studies (n > 400) conducted in the United States and Australia reported that 85% of women with PCOS were overweight or obese (93, 169). On the other hand, larger studies (n > 400) in China found that only approximately 20% of women with PCOS had a BMI of 25 kg/m² or greater (175, 176). When the Asian BMI cutoff for being overweight (BMI >23 kg/m² ) was used, the prevalence of overweight and obesity in China was found to be 35% (11), which was still notably lower than that seen in Western countries.
Body Fat Distribution
Women with PCOS are more likely to have upper body fat distribution compared with weight-matched controls (68, 177– 179). Greater abdominal or visceral adiposity is associated with greater insulin resistance, which could exacerbate the reproductive and metabolic abnormalities in PCOS. A study in the United Kingdom reported the highest prevalence of abdominal obesity, with 83% of the PCOS patients having a waist circumference above 88 cm (173). Studies in the United States and Italy have reported that around 65% of women with PCOS have abdominal obesity, as determined by having a waist circumference of more than 75th percentile by age or gender, or by having a waist/hip ratio of greater than 0.8 (171, 180). In China, a study noted that 31% of women with PCOS were abdominally obese, as determined by having a waist/hip ratio of 0.8 or greater (176). In contrast, a study in Japan found that 60% of women with PCOS had an upper-half/lower-half body fat ratio of 1.0 or greater (181). Differences in the determination of abdominal obesity limited any direct comparisons between the studies.Obesity in PCOS: Cause or Result
It is known that obesity is associated with PCOS, but its causal role in this disorder has yet to be determined. Very few studies have reported the effect of BMI on menstrual irregularity. These studies tend to find that obesity or being overweight is associated with a greater prevalence of oligomenorrhea or amenorrhea (182, 183). There also have been very few studies investigating the effect of body weight on hirsutism. Different scales were used to measure hirsutism in these studies. Studies that measured hirsutism using the Ferriman-Gallwey scores did not find a statistically significant association between BMI and hirsutism (184, 185). In terms of biochemical hyperandrogenism, most studies found that BMI was positively associated with the levels of total T, free T, or with the free androgen index (182, 186, 187). Excess body weight was also associated with decreased SHBG (182, 187). One study reported that greater BMI was associated with a greater number of ovarian cysts (185).Excess adiposity was also associated with poorer metabolic health in PCOS. Women with PCOS who had a greater BMI had higher fasting insulin levels and greater insulin resistance (175, 187, 188). Larger studies (n > 200) found that a greater BMI was associated with higher levels of LDL, triglyceride, and total cholesterol and lower levels of HDL (175, 188). Obesity and being overweight were also associated with higher blood pressure among women with PCOS (185, 188).
Fat distribution could have an effect on PCOS symptoms and metabolic health, independent of BMI. Several studies found that greater obesity in the central region as determined by waist circumference, visceral fat, or the central/peripheral fat ratio was associated with lower SHBG levels (171, 173, 189). Some studies found that a higher trunk/leg fat ratio or upper/lower region fat ratio is associated with higher levels of T (181, 190). The effect of fat distribution on hirsutism is unclear. One study reported that a greater proportion of women with upper obesity was hirsute compared with those with lower obesity, but the statistical significance level was not reported (191). The other two studies that looked at the effect of fat distribution on hirsutism did not find a statistically significant effect (180, 181).
Similarly, the effect of fat distribution on ovarian function is unclear. One study found that a greater proportion of women with PCOS who had upper region obesity had oligomenorrhea compared with those who had lower region obesity; the statistical significance level was not reported (191). The presence of ovarian cysts did not appear to be affected by fat distribution (180). Central obesity was associated with greater fasting insulin levels, greater insulin area under the curve, and greater insulin resistance (HOMA) (180, 192, 193). Central obesity was also associated with higher triglyceride levels (173, 180, 189) and possibly with lower HDL cholesterol levels (173, 180).
Ethnicity and Geography Affect Obesity
In our preliminary analysis of a selected group of articles, we found that the prevalence of being overweight or obese varied widely across geographic regions. It is unclear whether ethnicity plays a role in the relationship between obesity and PCOS. Some studies suggest that a higher BMI is associated with a greater prevalence of menstrual irregularity and hirsutism, but more studies are required to confirm this. Most studies found that a higher BMI was linked to higher androgen levels. Greater visceral adiposity appeared to be associated with greater insulin resistance, but its effect on menstrual irregularity and hirsutism is unclear. The relationship between central obesity and ovarian cysts has not been investigated in this pool of studies.In most studies, participants were recruited through convenience sampling (e.g., from an endocrine clinic of a hospital). As such, these studies were at high risk of selection bias. Studies were also heterogeneous in their definitions of PCOS, overweight or obese, abdominal obesity, hirsutism, and menstrual irregularity. This contributes to detection bias in these studies. Preliminary analysis suggests that regional differences exist in the prevalence of obesity in PCOS, which may have implications for the physiologic role of obesity in PCOS in these populations. This potential confounder needs to be taken into account in future studies in PCOS and obesity. Little is reported in terms of menstrual irregularity, hirsutism, and ovarian size and morphology, even though these were considered the defining features of PCOS according to the Rotterdam criteria.
Treatment with Lifestyle Interventions
Despite many uncontrolled papers on lifestyle interventions in obesity and PCOS (194, 195), there are relatively few randomized controlled studies—these are summarized in a recent Cochrane review (196) with percentage of change data (median change: -7.00%; 95% CI, -10.1, -3.90; P<.00001). There was no evidence of an effect for the lifestyle treatment compared with minimal treatment for BMI. Four studies reported on adiposity distribution and waist/hip ratio. There was a greater reduction in waist circumference (median: -1.95 cm; 95% CI, -3.34, -0.57; P=.006) and waist/hip ratio (median: -0.04; 95% CI, -0.07, -0.00; P=.02) for lifestyle treatment compared with minimal treatment. Reproductively, lifestyle modification improved total T levels (median: -0.27 nmol/L; 95% CI, -0.46, -0.09; P=.003) and the FerrimanGallwey score (median: -1.20; 95% CI, -2.36, -0.04; P=.04). Metabolically, lifestyle treatment improved the fasting insulin concentrations (median: -2.02 mIU/mL; 95% CI, -3.28, -0.77; P=.002) and the oral glucose tolerance test (OGTT) insulin (median -1,714.59 mIU/mL/minute; 95% CI, -2,122.50, -1,306.68; P<.00001). All controlled and uncontrolled evidence has recently been reviewed in a systematic fashion (88–90).INSULIN RESISTANCE AND INSULIN METABOLISM
Insulin resistance was first identified in women with PCOS diagnosed by the NIH criteria. It is a common but far from universal finding in this PCOS phenotype (197). Indeed, it was recognized very early on in the history of this association that women with other hyperandrogenic and/or polycystic ovary phenotypes, such as ovulatory women, did not have substantial insulin resistance (198, 199). This finding has been confirmed in numerous subsequent studies stratifying affected women according to the Rotterdam diagnostic criteria: women with the most marked metabolic abnormalities are those with hyperandrogenism and chronic anovulation (i.e., NIH criteria), independent of polycystic ovaries (89, 200–203). Other phenotypes have much milder metabolic dysfunction or are normal (204, 205). Moreover, women with polycystic ovaries according to the Rotterdam criteria and with regular cycles are metabolically normal, although they may have subtle hormonal abnormalities (43).Cellular Mechanisms of Insulin Resistance
The cellular and molecular mechanisms of insulin resistance in PCOS differ from those in other common insulin-resistant states such as obesity and T2D. There is a postbinding defect in insulin signaling associated with increased serine phosphorylation of the receptor and downstream signaling molecules (206). In vivo insulin action is profoundly decreased in the skeletal muscle secondary to signaling defects, but hepatic insulin resistance is present only in obese women with PCOS, indicating a synergistic negative effect of PCOS and obesity on insulin action (206). Pancreatic β-cell dysfunction is also present in PCOS but may be more related to T2D risk factors, as this dysfunction is most severe in women with a first-degree relative with T2D (207) and is associated with the strongest T2D risk allele, transcription factor 7-like 2 (TCF7L2) (208). Both insulin resistance and pancreatic β-cell dysfunction appear to be necessary for progression to T2D, as in other insulin-resistant states (209).Body Fat Distribution and Insulin Resistance
The association between upper body or android obesity and signs of androgen excess in women was first popularized by Vague (210), and women with increased upper body fat have increased production rates of androgens (211). Both obese and lean women with PCOS have increased upper body obesity, as determined by waist/hip girth ratios or waist circumference, compared with reproductively normal women of comparable weight (197, 212). However, in the limited number of studies that have assessed this parameter directly by computerized axial tomography or magnetic resonance imaging, visceral adipose mass has been similar in women with PCOS and reproductively normal women of comparable weight (213–215). Nevertheless, it remains possible that there are functional differences in PCOS adipose tissue, even if the absolute amount of fat mass does not differ from control women. For example, subcutaneous adipocytes in both lean and obese women with PCOS are larger than those from control women and have defects in insulin action and lipolysis (197, 216). Further, visceral adipocytes from women with PCOS have increased rates of lipolysis compared with those from control women (217), which could lead to increased portal levels of free fatty acids in PCOS even in the absence of increased visceral fat mass. Further characterization of adipocyte function, particularly visceral adipocytes, is an important area of future research in PCOS.Extensive evidence indicates that hyperinsulinemia contributes directly to dysreproductive function in PCOS (197). However, it is also clear that not all women with PCOS are insulin resistant. There is considerable evidence, both physiologic and genetic, that hyperandrogenism in PCOS is an intrinsic defect in PCOS (218). Because multiple PCOS phenotypes can be present within the same family, it would seem that insulin resistance is a modifying rather than a causative factor (218). Whether hyperandrogenism and insulin resistance in PCOS reflect the same genetic defect with variable expression, closely linked genes, or causally related abnormalities remains unknown. It is important to note that studies assessing the prevalence of insulin resistance in PCOS are limited by the accuracy of the method applied for quantifying insulin action. Moreover, there are also data to suggest that even in the absence of insulin resistance the ovarian response to insulin in PCOS may be abnormal (219).
Rebranding PCOS as a Unique Reproductive/ Metabolic Disorder
The metabolic syndrome (MetS) is a constellation of cardiovascular disease risk factors associated with insulin resistance (220). Women with classic NIH-criteria PCOS have significantly increased rates of MetS compared with reproductively normal women of similar age and weight (200, 221). This finding is consistent with the profound insulin resistance associated with PCOS. In addition, hyperandrogenism may be an independent contributor to MetS risk (206). Further, endogenous androgen levels are higher in women with MetS in the absence of PCOS (222). Blocking androgen action or improving insulin sensitivity can improve features of MetS in PCOS (223).It is now widely appreciated that PCOS is a metabolic as well as a reproductive disorder. The long-term impact of PCOS-associated metabolic disorders remains poorly studied, but it is clear that they are a major contributor to prediabetes, diabetes, and MetS in reproductive-age women. One of the aims of Rotterdam was to identify and characterize additional phenotypes. The weight of the evidence indicates that not all PCOS phenotypes have similar metabolic risk. It is critical for public health and for the design of long-term studies to stratify women with PCOS according to metabolic risk. This goal would be greatly facilitated by using a specific name for this high-risk PCOS subset that would differentiate it from those women with a purely reproductive phenotype. The name Syndrome XX has been proposed (206), but other names, such as Female Metabolic Syndrome (FMS) or Metabolic Reproductive Syndrome (MRS), may also be appropriate.
TYPE 2 DIABETES
Metabolic Phenotype of PCOS
The first reports of metabolic abnormalities in PCOS date back some 30 years, and it is now well established that PCOS is characterized by peripheral insulin resistance (affecting postreceptor insulin signaling) and compensatory hyperinsulinemia (197, 198, 224, 225). Polycystic ovary syndrome is a heterogeneous syndrome, and metabolic abnormalities are most often seen (but are not invariable) in women who have both hyperandrogenism and anovulation (152, 153, 198, 199, 226, 227). Overweight status and obesity amplify metabolic dysfunction, but insulin resistance may be observed even in lean women with PCOS (197, 198).PCOS and Diabetes
Given the frequent occurrence of insulin resistance, it is not surprising that PCOS is associated with impaired glucose tolerance and T2D, and the syndrome is now considered to be a significant risk factor for development of T2D both in later life and, increasingly, in overweight or obese young women with PCOS. Figures regarding the prevalence of impaired glucose tolerance (IGT) and T2D in PCOS vary quite widely. Studies of clinic populations of women with PCOS indicate that between 10% and 45% have either IGT or overt T2D (228–230). Larger, epidemiologic studies indicate that the odds ratio for development of diabetes in women with PCOS (or at least with symptoms of PCOS) is around 2.0 after adjustment for BMI but between 2.8 and 3.8 if BMI is included (231–233). Obesity, by amplifying insulin resistance, is a confounding factor in the development of IGT and T2D in women with PCOS, but the increasing prevalence of obesity in the population means that a further increase in the prevalence of diabetes is to be expected. In a recent meta-analysis reviewing 2,192 studies, 35 of which were selected for further analysis, the odds ratio for IGT was approximately 2.5 and for T2D was 4.0 in BMI-matched groups (89).Few studies have examined the conversion of IGT to diabetes in the same cohort of patients, but one longitudinal study reported a change in the prevalence of IGT from 37% to 45% and of T2D from 10% to 15% over a follow-up period of 2 to 3 years (202). Another study observing a cohort of women with PCOS for 6.2 years showed a conversion rate from IGT to diabetes of 54% (234).
Gestational Diabetes
Gestational diabetes (GDM) is also linked to PCOS. There have been no large studies of prevalence to date, but a recent metaanalysis suggests that there is an overall threefold increase in risk in women with PCOS (126). Etiology of IGT and T2D in Women with PCOS
There is ample evidence for a genetic basis of PCOS, and it is clear that abnormalities of glucose/insulin homeostasis are more common in first-degree relatives of women with PCOS than in a reference population (235, 236). It is therefore logical to consider genes that have recently been identified as contributing to the genotype and phenotype of T2D as candidate genes in the etiology of PCOS. The evidence that such genes play a part in the genetic etiology of PCOS is somewhat conflicting (205, 208, 214, 215). One confounding factor in the interpretation of the current evidence is that women with PCOS and their relatives who are known to have either IGT or T2D are typically excluded from the analysis.Risk Factors for T2D in PCOS
As previously above, it is those women who have both androgen excess and anovulation who are most vulnerable to developing metabolic dysfunction, including IGT and T2D. Introduction of the broader (Rotterdam) diagnostic criteria for PCOS appropriately accommodates the heterogeneous nature of the syndrome (symptoms may change even within the same patient). It is interesting (and important from a prognostic viewpoint) that it appears that women with only polycystic ovaries, or PCOS with anovulation but without androgen excess, or hyperandrogenism but regular menses usually have normal insulin sensitivity and metabolic status—and presumably are not at the same increased risk of development of IGT or T2D compared with those with the ‘‘classic’’ features of the syndrome (152, 153, 226, 227). Other obvious factors that contribute to the risk of diabetes are obesity and a family history of T2D (207).Management of IGT and T2D in PCOS
As yet there are no large-scale interventional studies that specifically relate to the treatment of IGT or T2D in women with PCOS. As in all patients with these disorders, diet and lifestyle changes, and treatment with metformin, insulin-sensitizing drugs, and other oral hypoglycemic agents may be considered (122). However, there is already concern about the long-term safety of thiazolidinediones, and these agents should be used with extreme caution or arguably avoided altogether in women of reproductive age. Preliminary studies of the efficacy of bariatric surgery in reversing metabolic (and indeed endocrine) abnormalities in women with PCOS have produced encouraging results (237).CARDIOVASCULAR HEALTH: MARKERS
Cardiovascular disease (CVD) is caused by genetic and lifestyle influences. Nine risk factors account for 94% of the population attributable risk for coronary heart disease (CHD) in 52 different countries (238). The odds for an event are 2.5 to 5.0 times higher with each of these risk factors. The findings are consistent at all ages and in all regions. Roughly 1% to 8% of myocardial infarctions (MI) occur in individuals <40 years. Most occur in women who are ages 50, 60, or 70 years. Increased risk is associated with hypertension, diabetes, abdominal obesity, psychological factors, smoking, and altered circulating apoA1/ApoB ratios. Exercise, consumption of adequate fruits and vegetables, and alcohol are negative risk factors for MI. The prevalence of each risk factor is roughly double for women with PCOS when compared with controls, is 1.5 times higher in BMI-matched studies beginning in adolescence, and is found in every decade.Relatively little work has been done regarding the effects of diet on metabolic risk in women with PCOS. It was found that women with PCOS, compared with controls, were more likely to eat foods with a higher glycemic index (239). One randomized trial found reduced inflammatory markers in patients with PCOS when given a low glycemic diet compared with a conventional diet when used in conjunction with metformin (240). The hormone responses may differ with Western vs. low-fat, high-fiber diets (241). In overweight women with PCOS, weight loss improves arterial compliance and postprandial lipidemia (90). Modifying dietary carbohydrate or protein content in weight-loss programs provided similar improvements in arterial compliance and postprandial lipidemia.
Psychosocial morbidity is very prevalent in PCOS (242). Quality of life is commonly compromised by depression and/or anxiety (242). A recent meta-analysis (243) found that the odds of prevalent depression are four times higher in women with PCOS compared with non-PCOS controls. The odds were still four times higher even when women with PCOS were BMI-matched to non-PCOS controls. Depression is therefore an important risk factor for CVD.
In normal-weight women with PCOS compared with BMI-matched controls (BMI: 21) more android central fat distribution occurs (178). In lean women with PCOS compared with lean controls, increased catecholamine-induced lipolysis and altered hepatic lipase are present (217, 244). The severity of insulin resistance is related to the amount of abdominal obesity in women with PCOS, even when they are not obese (192). This is likely to be at least in part instrumental in their propensity to develop impaired glucose tolerance, metabolic syndrome, and/or diabetes mellitus. The CVD risk markers are magnified by obesity (90, 245, 246). The odds of prevalent diabetes are approximately three times higher in women with PCOS compared with women who do not have PCOS; in BMI-matched studies, the odds are approximately double (89). The prevalence of MetS differs by geographic region (165, 175) and phenotypic variation in the diagnosis of PCOS. The more severe phenotypes are associated with more metabolic syndrome, and this has been found in obese and nonobese women (175, 247). The set point for when central obesity becomes associated with MetS may be different in different ethnic and racial groups (175, 248).
Dyslipidemia is common in women with PCOS (249). Although the most common dyslipidemic pattern was thought to be elevated triglycerides and low HDL cholesterol, most patients with PCOS have normal triglyceride levels, albeit higher when women with PCOS are matched with women who do not have PCOS. This has been found whether the studies used NIH or Rotterdam criteria or included obese or nonobese women. On average, women with PCOS had mean triglyceride concentrations that were 30 mg/dL higher by NIH criteria or 18 mg/ dL higher by Rotterdam criteria. The most revealing lipid abnormality was higher non-HDL cholesterol in women with PCOS. This reflects altered ApoB/A1 ratios, an important risk factor for CVD. Altered ApoB/ApoA ratios reflect a more atherogenic lipoprotein lipid pattern in women with PCOS. The LDL-cholesterol concentration is mildly elevated in women with PCOS, and this probably reflects the measurement of increased total LDL due to a greater production of more atherogenic small LDL particles in women with PCOS (250). These differences occur in women with PCOS compared with non-PCOS controls even when they are lean. The differences also occur with greater magnitude in obese women with PCOS. This suggests that excess weight is an effect modifier for apoprotein lipid abnormalities in women who have PCOS.
Whether insulin resistance or androgen excess causes the altered apolipoprotein lipid changes needs more investigation. Abdominal obesity is associated with more fatty acids circulating into the portal vein. In states of insulin resistance, Apo CII/III ratios are altered in women with PCOS (251). This leads to a greater production of triglycerides, which are metabolized to more atherogenic circulating small LDL particles that enter into the arterial subendothelial space. Atherogenesis is an inflammatory disorder that occurs in response to these circulating small LDL particles entering the subendothelial space in the vascular system (252)—particularly in areas of high shear stress.
Systemic inflammation is commonly present in women with PCOS (253). Numerous biochemical inflammatory and thrombotic markers of CVD risk circulate in excess in women with PCOS (247). Tumor necrosis factor-a (TNF-a), interleukin-6 (IL-6), IL-18, IL-17, factor VIIc, tissue plasminogen activator (t-PA), fibrinogen, von Willebrand factor (vWF), plasminogen activator inhibitor-1 (PAI-1), thrombomodulin, D-dimers, antithrombin III (ATIII), Sp-Selectin, endothelin-1 (ET-1), asymmetric dimethylarginine (ADMA), intercellular adhesion molecule-1 (ICAM-1), soluble vascular cell adhesion molecule-1 (sVCAM-1), serum advanced glycation end-products (AGEs), membrane receptor for AGEs (RAGE), malondialdehyde (MDA), nitric oxide (NO), latencyassociated peptides (LAPs) compared with non-PCOS controls. Of strong interest is elevated ADMA, a factor that is thought to mediate nitrous oxide activity, which is instrumental to vascular constriction and oxidation. Levels of ADMA were found to be elevated in women with PCOS even when matched to similar-weight obese non-PCOS controls (254); and this correlated with insulin resistance.
Arterial stiffness has been reported to be worse in nonobese women with PCOS compared with controls (255). Fifteen of 24 studies (NIH or Rotterdam criteria) showed significantly lowered flow-mediated dilation in women with PCOS when compared with controls. Common limitations are that the studies included only Caucasian women, had small sample sizes, and used different definitions of PCOS. Most studies did not address physical activity and did not include smoking as a confounder. Fourteen of 23 cross-sectional comparison studies showed a significantly higher carotid intima-media thickness in women with PCOS as compared with controls (249). For each of these markers of CVD in women with PCOS, it would be desirable to have larger studies, with clearer definitions of PCOS and more commonly agreed upon methodology and reporting of results.
Women with classic PCOS are more likely to have prevalent T2D, IGT, and/or MetS (89). In BMI-matched studies, the odds of prevalent disease are 2.5 times higher. One of the ways in which women with PCOS may have a greater risk for CVD is through the development of diabetes. The metabolic syndrome is a categorical construct to convey that having four of five components is associated with a relative risk of 44 for the development of diabetes (250). Polycystic ovary syndrome is now a well-defined risk factor for CVD. Although the term ‘‘metabolic syndrome’’ is a categorical measure, in reality there is a continuum of deleterious effects reflected for any of the individual components.
Cardiovascular disease is a progressive disorder starting in childhood. Most studies of CVD risk factors have compared women with PCOS to controls in their second decade of life (earlier than the usual horizon for events), but they have nevertheless demonstrated more adverse changes in women with PCOS. However, these same risk factors (abdominal obesity, altered apoA1/apoB ratios, and glucose abnormalities) have been found in women with PCOS compared with matched non-PCOS controls in their seventh decade of life as well (256). Of interest is that having PCOS was a stronger risk factor for CHD than was age (256) in postmenopausal women. In early postmenopausal women, Maturana et al. (257) found a correlation between high-sensitivity C reactive protein (hs-CRP) and endothelin-1 (a marker of vascular damage) and T, after adjustment for insulin resistance.
Conclusion
Women with PCOS cluster several CVD risk markers. A registry for patients with CVD and a multisite longitudinal follow-up study or case-control study is urgently needed. This would use well-defined cohorts to provide better accuracy and more precise estimates of the magnitude of CVD risk related to PCOS and to correlate numerous markers of atherosclerotic changes to CVD events. Further study is needed to clarify each new marker and to determine how the changes they reflect are related to the common universal risk factors for CVD. These studies hopefully can provide information on whether these newly discovered markers provide added benefit to CVD risk prediction. In addition, they should provide information as to whether PCOS status per se is a risk factor of CVD, and the modifying effect of various lifestyle factors.CARDIOVASCULAR HEALTH: OUTCOMES
Life-long metabolic dysfunction in women with PCOS exaggerates the risk for CVD with aging, particularly after menopause. This metabolic dysfunction is based upon insulin resistance, which occurs in most women with PCOS (258), and is independent and additive with obesity (259). Consequently, beginning in adolescence, impaired glucose tolerance (IGT) and T2D are highly prevalent in women with PCOS (odds ratio approximately 4:1) (229, 230, 260) and occur in about 40% of women with PCOS by the fourth decade of life, with age and weight gain worsening glycemic control (203, 234, 261).Insulin-resistant women with PCOS have vascular dysfunction, which is associated with total as well as abdominal adiposity (262, 263). Women with PCOS also have more subclinical vascular disease than normal women, adjusting for age and BMI. Retrospective studies show that increased waist/hip ratio, hirsutism, or polycystic ovaries more commonly accompany stenotic coronary artery disease in women undergoing coronary angiography (246, 264). Casecontrol studies further show that the severity of carotidintima media thickness (265, 266), coronary artery calcification, and to a lesser extent aortic calcification (267– 269) also are greater in women with PCOS than controls by NIH criteria, independent of age and BMI. These markers of subclinical atherosclerosis are correlated with age, components of MetS, insulin resistance, and circulating androgen levels (265, 266, 269), with surgical or natural menopause being an independent risk factor for coronary artery calcification as well (269).
Nevertheless, evidence for increased CVD morbidity and mortality in women with PCOS remains inconclusive, given the epidemiologic challenges of case ascertainment by ovarian morphology and/or clinical symptoms, variable effect of adiposity, effects of medical and/or surgical therapies on cardiovascular outcomes, and low prevalence of CVD in young women. Four studies have used Rotterdam and/or NIH criteria to define PCOS and have observed PCOS cases for CVD end points, with study designs being either cross-sectional using small numbers (270, 271) or prospective with larger numbers (232, 233, 272). Cross-sectional studies with small patient numbers have reported either a higher prevalence of self-reported diabetes (32% PCOS, 8% controls) and coronary artery disease (21% PCOS, 5% controls) in 28 middle-aged women with polycystic ovaries at surgery for infertility; oligomenorrhea and hirsutism compared with 752 agematched controls (271); or a higher prevalence of diabetes but not cardiac disease in 346 similarly aged women with PCOS compared with a national survey of the Dutch female population (270). One prospective cohort study of 82,439 female nurses from the Nurses’ Health Study showed that women reporting menstrual irregularity at age 20 to 35 years (presumably from PCOS) had an increased risk for nonfatal (OR 1.25; CI 1.07–1.47) and fatal (OR 1.67; CI 1.35–2.06) CHD versus those with regular menstrual cycles, independent of age, BMI, and other confounders (57). Other prospective studies, however, did not confirm an increased prevalence of nonfatal/fatal CHD in women with PCOS (232, 233, 272), defined by histologic evidence of polycystic ovaries and clinical findings of hyperandrogenism and ovulatory dysfunction, although the risk for nonfatal cerebrovascular disease was higher in women with PCOS (232, 233).
To assess the PCOS-related risk for CVD in later life, some studies have attempted to diagnose PCOS in postmenopausal women by various criteria. A recent substudy of the Women’s Ischemia Evaluation Study (WISE) (273) confirmed that postmenopausal women with premenopausal menstrual irregularity and present hyperandrogenemia have a larger number of cardiovascular events than other postmenopausal women. In this study, multivessel cardiovascular disease occurred in 32% of PCOS-like women compared with 25% of nonPCOS-like women (OR 1.7; 95% CI, 1.1–2.8, adjusted for age and BMI), and correlated with diabetes, hypertriglyceridemia, and increased free T. Moreover, the event-free survival (including fatal and nonfatal events) was statistically significantly lower in women with PCOS compared with controls (hazard ratio 1.6; 95% CI, 1.2–2.1), controlling for age, diabetes, BMI, and angiographic coronary artery disease. It is interesting that the difference between the two groups was even higher when cerebrovascular accidents were included, confirming the association of PCOS with stroke. In addition, a cross-sectional study of 713 postmenopausal women (mean age: 73.8 years) found in nondiabetic women with intact ovaries a stepwise, graded association between CVD and the number of features of PCOS, including premenopausal menstrual irregularity, hirsutism, and postmenopausal biochemical hyperandrogenism (256). Another case-control study of 414 postmenopausal women (mean age: 60.4 years) confirmed in women with a history of premenopausal menstrual irregularity (as a surrogate marker of PCOS) an increased odds ratio for CVD (274). As an important side note, these studies emphasize the technical challenges of accurately measuring low circulating androgen levels after menopause.
Conclusion
Collective data suggest that surrogate markers of cardiovascular risk are higher in PCOS, adjusted for age and BMI. Studies of subclinical cardiovascular disease further show that endothelial dysfunction in PCOS is related to insulin resistance and total as well as abdominal obesity, while coronary artery calcification/carotid intima media wall thickness also are increased in PCOS compared with matched controls. Nevertheless, the degree to which surrogate markers of cardiovascular risk and subclinical cardiovascular disease correlate with cardiovascular events in PCOS remains unclear. Although uncertainty exists as to whether PCOS per se increases cardiovascular mortality, atherosclerotic CVD in postmenopausal women is associated with several PCOS-like features. Therefore, lifelong metabolic dysfunction in women with PCOS exaggerates CVD risk, causing a possible increase in CVD events with age, especially after menopause.CANCER RISK
Polycystic ovary syndrome is a common reproductive disorder resulting in a disruption of normal reproductive physiology that putatively may be associated with increased risk of the development of cancer of the endometrium, ovary, or breast, directly or mediated by its associated reproductivemetabolic alterations.It is well accepted that unopposed estrogen, secondary to anovulation, is a risk factor for the development of endometrial cancer. The magnitude of the risk has not been precisely quantified, but the best estimate based on a moderate amount of data is an odds ratio of approximately 2.7 (275). There is also limited information to suggest that women with PCOS have an elevated risk of ovarian cancer (relative risk approximately 2.5) (276). Conversely, there are limited data to suggest that there is no association of PCOS with breast cancer. There is insufficient evidence to evaluate any association of PCOS with vaginal, vulvar, or cervical cancer.
When assessing the association of PCOS with the risk of cancer, it is difficult to separate the cancer risk associated with other recognized risk factors such as nulliparity, infertility, treatment of infertility, anovulation, and obesity. Because of this confounding effect as well as a paucity of welldesigned research on the subject, the estimates provided for the strength of association are likely sensitive to a number of factors including [1] lack of precision of the estimate due to a small number of studies and a small number of cases in each study; [2] limitations in the definition of PCOS, including that the definition has changed over time, that the diagnoses used in many of the studies are based on self-report, and that anovulation may have been used as a surrogate diagnosis for PCOS; [3] limitations in comparison (control) populations, including lack of generalizability as some studies have included or excluded women with infertility in comparison populations, and the prevalence of PCOS is often low in control populations.
Endometrial Cancer
Although the degree of risk has not been clearly defined, it is generally accepted that women with PCOS who experience symptoms of amenorrhea are at greater risk for endometrial hyperplasia and cancer. However, the data to support the general acceptance are not definitive. Most of the data are based on case series with few longitudinal studies with a comparison group. The increased risk is thought to be related to unopposed endogenous estrogen, but may also be related to elevated androgens.A three times increased risk of endometrial cancer was shown in patients with chronic anovulation (277). In addition, a possible association of PCOS and endometrial cancer remained uncertain (278). It was speculated that there might be a differential risk, especially for subgroups of women, possibly those who are obese. A meta-analysis on this subject included four studies(275). Three showed elevated risk, although only one was statistically significant, and one showed no risk.
Among 4,056 women there were 666 cases of endometrial cancer, of which 29 were diagnosed with PCOS. In the 3,379 controls, 27 women were diagnosed with PCOS. The aggregate analysis demonstrated an odds ratio of 2.7 (1.0–7.29). This translates into an increase in risk from 17 to 46 per 100,000 women (275). Some limitations in combining the data include different age groups used for inclusion criteria. Some studies were population based while others used cancer registries. A greater strength of association has been noted with an odds ratio of 5.3 (1.5–18.6) for all women with PCOS and 6.1 (1.0–36.9) for those women with PCOS with an elevated BMI (232, 233). However, this study was crosssectional and was not included in the meta-analysis. Most cancers have been found to be well differentiated and have a good prognosis. There has been no histologic difference noted in endometrial cancers in women with and without PCOS.
Preventative measures for endometrial cancer include recognition and treatment of unopposed estrogen with periodic progestogen withdrawal and/or surveillance with endometrial biopsy for those with extended periods of amenorrhea. It is recommended that women should have at least four withdrawal bleeding episodes per year (every 3 months). Surveillance with endometrial biopsy or ultrasound to assess endometrial thickening for those with extended periods of amenorrhea is recommended based on clinical suspicion and presentation.
Ovarian Cancer
Putative theories for an increase in the risk of ovarian cancer include ‘‘incessant’’ ovulation. If this theory is true, one may hypothesize that women with PCOS may have a lower risk of cancer. However, ovarian cancer may be associated with infertility and/or nulliparity, which may increase the risk of ovarian cancer. There are very few studies that have evaluated the risk of ovarian cancer in women with PCOS. In 1979, McGowan et al. (279) demonstrated a 2.5-fold increased risk was demonstrated in nulliparous women in respect to ovarian cancer. Moreover, a sevenfold increase was demonstrated in nulliparous women with infertility-related anovulation, but it is not clear that these women truly had PCOS. Only one large study evaluated the association of histologically confirmed epithelial ovarian cancer with women who reportedly had PCOS (276). Of the 4,547 women in the study, there were 476 cases of ovarian cancer, of which 7 were in conjunction with PCOS. Of the 4,081 controls, 24 had a PCOS diagnosis. The calculated odds ratio was 2.52 (1.08–5.89), which translates into a risk of 17.4 to 44 per 100,000 women. Critiques of the Schildkraut et al. (276) study include that this association was not a primary aim of the study and that the seven cases of PCOS accounted for only 1.5% of the group of women with cancer. The 27 cases of PCOS in the control accounted for only 0.06% of the controls. Underrepresentation of women with PCOS in the control group may have biased the study.Corroborating evidence of an increased risk of ovarian cancer may be found in a few older, small studies (280– 283), which demonstrated that clomiphene citrate treatment is associated with a 2.3-fold increase in ovarian cancer and perhaps is a surrogate marker of cancer in women with PCOS. Potentially contradictory evidence for the risk of ovarian cancer in women with PCOS was provided by a study that documented a standardized mortality rate for ovarian cancer in PCOS that was lower than in the women without PCOS (SMR 0.39; 95% CI, 0.01 to 2.17) (272). Use of combined OCPs is known to be a preventive measure for ovarian cancer risk. Treatment of infertility resulting in live birth is also thought to be preventative. There is no recommended routine surveillance strategy for the detection of ovarian cancer in women with PCOS.
Breast Cancer
There are limited data evaluating the risk of breast cancer in women with PCOS. Putative risk factors for breast cancer include obesity and nulliparity, which are common findings in PCOS. A meta-analysis of three studies was performed (275). Of the studies included, one showed a trend to an increased risk, one showed protection, and one showed no risk. In aggregate, no association was found. In total, the studies included 23,842 women: 11,836 cases of breast cancer, of which 59 were diagnosed with PCOS. Of the 12,006 controls, 74 had PCOS. This resulted in an odds ratio of 0.88 (0.44–1.77). Limitations of the analyses include that clinical characteristics of both groups varied in the studies and the diagnosis of breast cancer was based on recall of a physician’s diagnosis in some studies. Cancer cases were pooled together irrespective of stage and histology. This study was thus unable to address whether there were differences in histology, type, or stage of the breast cancer. Other limitations included that women with PCOS accounted for less than 1% of the study control population and for under 0.5% in the breast cancer group in one study, and for only 1.35% in a second. Also, PCOS was reported by patients and was not documented.Standardized mortality rates for a 30-year follow-up evaluation for all neoplasms in women with PCOS was 0.91 (95% CI, 0.6–1.32). It was 1.48 (95% CI, 0.78–2.54) for women with PCOS diagnosed with breast cancer (272). Currently, there are no recommended alterations in clinical care to assess a differential risk of breast cancer in women with PCOS.
MENOPAUSE, GENERAL HEALTH, LONGEVITY
Menopause The transition of women with PCOS into menopause and what the phenotype of PCOS in postmenopause may be is poorly understood. There is evidence that women with PCOS have a larger cohort of primary follicles than age-matched control women based on histologic evaluation (284), antral follicle counts (285), and levels of serum AMH (286). Further, serum levels of AMH tend to remain persistently elevated over time in women with PCOS (287). These findings suggest prolonged reproductive function in these women as well as increased ovarian reserve. Studies in the normal female population (288) and women with PCOS (289) have shown a marked decrease in serum T levels as women age from the third to fifth decades, proportionately greater than during the perimenopause to menopausal transition. Additionally, women with PCOS develop improved menstrual regularity with age (37), and most predictive models for pregnancy during ovulation induction in women with PCOS show that age increases the chance of ovulation (despite being associated with lower pregnancy rates) (290, 291). These may all contribute to improved reproduction with aging. Lifetime fecundity in a Scandinavian population of women with PCOS was comparable with that of a control group, although there was increased use of infertility treatments (285). There is evidence from at least one prospective cohort study that evaluated women through menopause that women with PCOS may experience menopause on average 2 years later than healthy control women (42).There is no known menopausal PCOS phenotype, despite multiple attempts to create one, usually with a mixture of current and past phenotypic characteristics. The criterion that seems most likely to normalize at menopause is the polycystic ovary, at least in terms of follicle count and volume, both of which tend to decrease with age in all women (48). Some studies using existing menopausal women have relied on a prior history of irregular menses (256), the presence of polycystic ovaries (292), or current hyperandrogenemia or hirsutism (often compared with menopausal controls) (273). However, testosterone assays, even those using mass spectrometry, have poor precision in the menopausal range and thus are likely to confound the diagnosis (293). Unfortunately, phenotype drift is common in such situations, and expanded criteria including an elevated waist circumference or evidence of biochemical insulin resistance have been added in at least one study (256). These are likely to elevate the true prevalence of PCOS in such studies.
General Health
The general health and best treatment of postmenopausal women with PCOS are poorly understood; one study referred to this area as a ‘‘black hole’’ in medicine (294). It is suspected that women with PCOS have increased rates of obesity and diabetes in menopause (36), which are likely to decrease their quality of life. Although data are conflicting, most reports tend to show normal or increased bone mineral density in women with PCOS (295, 296), but changes during menopause have not been described. As noted in other sections, women with PCOS have multiple risk factors for CVD and may be at increased risk for premature CVD events. Some aspects of PCOS associated with cardiovascular risk may plateau with age, such as LDL cholesterol levels (297) or glucose tolerance (203). Thus, without appropriate longitudinal follow-up observations, it is incorrect to assume that age will exacerbate outcomes in women with PCOS. Other aspects of general health that have been studied in younger women with PCOS, such as mood (86, 243) or sleep disorders (298), have not been studied in postmenopausal women with PCOS. Hirsutism is a common complaint of postmenopausal women, and alopecia is more common with aging. However, the natural history of hirsutism and/or alopecia in postmenopausal women with PCOS is unknown. A well-designed trial of 5a-reductase inhibition in postmenopausal women with androgenic alopecia showed no benefit (299) (whereas it is effective in males with androgenic alopecia), which suggests that other factors are contributory in women.Longevity
It is difficult from the existing data to know what the effect of the diagnosis of PCOS is on mortality. Retrospective data in women with polycystic ovaries suggest mortality occurs at a similar rate as in the general population (and presumably at the same age) (232, 233). The best prospective data on postmenopausal women diagnosed with suspected PCOS suggest they have increased CVD event rates and decreased survival (273). Limited family studies have suggested increased and earlier cardiovascular event rates in the mothers and fathers of women with PCOS (300, 301).REFERENCES
- Carmina E, Oberfield SE, Lobo RA. The diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol 2010;203:201–5.
- Shayya R, Chang RJ. Reproductive endocrinology of adolescent polycystic ovary syndrome. BJOG 2010;117:150–5.
- Sultan C, Paris F. Clinical expression of polycystic ovary syndrome in adolescent girls. Fertil Steril 2006;86(Suppl 1):S6.
- Olutunmbi Y, Paley K, English JC III. Adolescent female acne: etiology and management. J Pediatr Adolesc Gynecol 2008;21:171–6.
- Jeffrey CR, Coffler MS. Polycystic ovary syndrome: early detection in the adolescent. Clin Obstet Gynecol 2007;50:178–87.
- Blank SK, Helm KD, McCartney CR, Marshall JC. Polycystic ovary syndrome in adolescence. Ann NY Acad Sci 2008;1135:76–84.
- Metcalf MG, Skidmore DS, Lowry GF, Mackenzie JA. Incidence of ovulation in the years after the menarche. J Endocrinol 1983;97:213–9.
- Venturoli S, Porcu E, Fabbri R, Paradisi R, Ruggeri S, Bolelli G, et al. Menstrual irregularities in adolescents: hormonal pattern and ovarian morphology. Horm Res 1986;24:269–79.
- Wiksten-Almstromer M, Hirschberg AL, Hagenfeldt K. Prospective follow-up of menstrual disorders in adolescence and prognostic factors. Acta Obstet Gynecol Scand 2008;87:1162–8.
- van Hooff MH, Voorhorst FJ, Kaptein MB, Hirasing RA, Koppenaal C, Schoemaker J. Predictive value of menstrual cycle pattern, body mass index, hormone levels and polycystic ovaries at age 15 years for oligo-amenorrhoea at age 18 years. Hum Reprod 2004;19:383–92.
- Venturoli S, Porcu E, Fabbri R, Pluchinotta V, Ruggeri S, Macrelli S, et al. Longitudinal change of sonographic ovarian aspects and endocrine parameters in irregular cycles of adolescence. Pediatr Res 1995;38:974–80.
- Mortensen M, Rosenfield RL, Littlejohn E. Functional significance of polycystic-size ovaries in healthy adolescents. J Clin Endocrinol Metab 2006;91:3786–90.
- Herter LD, Magalhaes JA, Spritzer PM. Relevance of the determination of ovarian volume in adolescent girls with menstrual disorders. J Clin Ultrasound 1996;24:243–8.
- McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab 2009;94:56–66.
- Blok GJ, de Boer H, Gooren LJ, van der Veen EA. Growth hormone substitution in adult growth hormone-deficient men augments androgen effects on the skin. Clin Endocrinol (Oxf) 1997;47:29–36.
- Messenger AG. Thyroid hormone and hair growth. Br J Dermatol 2000;142:633–4.
- Shapiro J, Lui H. Vaniqa—eflornithine 13.9% cream. Skin Ther Lett 2001;6: 1–3, 5.
- Speroff L, Glass R, Kase N. Hirsutism. In: Weinberg RW, Murphy J, Pancotti R, editors. Clinical gynecologic endocrinology and infertility. Baltimore: Lippincott, Williams & Wilkins; 1999:523–56.
- Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology 2003;206:57–67.
- Azziz R. The time has come to simplify the evaluation of the hirsute patient. Fertil Steril 2000;74:870–2.
- Harborne L, Fleming R, Lyall H, Sattar N, Norman J. Metformin or antiandrogen in the treatment of hirsutism in polycystic ovary syndrome. J Clin Endocrinol Metab 2003;88:4116–23.
- Essah PA, Wickham EP III, Nunley JR, Nestler JE. Dermatology of androgen-related disorders. Clin Dermatol 2006;24:289–98.
- Lowenstein EJ. Diagnosis and management of the dermatologic manifestations of the polycystic ovary syndrome. Dermatol Ther 2006;19:210–23.
- Futterweit W, Dunaif A, Yeh HC, Kingsley P. The prevalence of hyperandrogenism in 109 consecutive female patients with diffuse alopecia. J Am Acad Dermatol 1988;19:831–6.
- Cela E, Robertson C, Rush K, Kousta E, White DM, Wilson H, et al. Prevalence of polycystic ovaries in women with androgenic alopecia. Eur J Endocrinol 2003;149:439–42.
- Kaufman KD. Androgen metabolism as it affects hair growth in androgenetic alopecia. Dermatol Clin 1996;14:697–711.
- Sawaya ME, Price VH. Different levels of 5a-reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. J Invest Dermatol 1997;109:296–300.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004;19:41–7.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25.
- Hart R, Hickey M, Franks S. Definitions, prevalence and symptoms of polycystic ovaries and polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol 2004;18:671–83.
- Laven JS, Imani B, Eijkemans MJ, Fauser BC. New approach to polycystic ovary syndrome and other forms of anovulatory infertility. Obstet Gynecol Surv 2002;57:755–67. 38.
- Azziz R, Ehrmann D, Legro RS, Whitcomb RW, Hanley R, Fereshetian AG, et al. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo-controlled trial. J Clin Endocrinol Metab 2001;86:1626–32.
- Kumarapeli V, Seneviratne RA, Wijeyaratne CN, Yapa RM, Dodampahala SH. A simple screening approach for assessing community prevalence and phenotype of polycystic ovary syndrome in a semi-urban population in Sri Lanka. Am J Epidemiol 2008;168:321–8.
- Apter D. Endocrine and metabolic abnormalities in adolescents with a PCOS-like condition: consequences for adult reproduction. Trends Endocrinol Metab 1998;9:58–61.
- Bekx MT, Connor EC, Allen DB. Characteristics of adolescents presenting to a multidisciplinary clinic for polycystic ovarian syndrome. J Pediatr Adolesc Gynecol 2010;23:7–10.
- Dahlgren E, Johansson S, Lindstedt G, Knutsson F, Oden A, Janson PO, et al. Women with polycystic ovary syndrome wedge resected in 1956 to 1965: a long-term follow-up focusing on natural history and circulating hormones. Fertil Steril 1992;57:505–13.
- Elting MW, Korsen TJ, Rekers-Mombarg LT, Schoemaker J. Women with polycystic ovary syndrome gain regular menstrual cycles when ageing. Hum Reprod 2000;15:24–8.
- Elting MW, Kwee J, Korsen TJ, Rekers-Mombarg LT, Schoemaker J. Aging women with polycystic ovary syndrome who achieve regular menstrual cycles have a smaller follicle cohort than those who continue to have irregular cycles. Fertil Steril 2003;79:1154–60.
- Laven JS, Mulders AG, Visser JA, Themmen AP, de Jong FH, Fauser BC. Anti-mullerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab 2004;89: 318–23.
- de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril 2002;77:357–62.
- Visser JA, de Jong FH, Laven JS, Themmen AP. Anti-mullerian hormone a new marker for ovarian function. Reproduction 2006;131:1–9.
- Tehrani FR, Solaymani-Dodaran M, Hedayati M, Azizi F. Is polycystic ovary syndrome an exception for reproductive aging? Hum Reprod 2010;25: 1775–81.
- Vutyavanich T, Khaniyao V, Wongtra-Ngan S, Sreshthaputra O, Sreshthaputra R, Piromlertamorn W. Clinical, endocrine and ultrasonographic features of polycystic ovary syndrome in Thai women. J Obstet Gynaecol Res 2007;33:677–80.
- Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, West C, et al. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod 1995;10:2107–11.
- Diamanti-Kandarakis E, Panidis D. Unravelling the phenotypic map of polycystic ovary syndrome (PCOS): a prospective study of 634 women with PCOS. Clin Endocrinol (Oxf) 2007;67:735–42.
- Taponen S, Martikainen H, Jarvelin MR, Laitinen J, Pouta A, Hartikainen AL, et al. Hormonal profile of women with self-reported symptoms of oligomenorrhea and/or hirsutism: Northern Finland birth cohort 1966 study. J Clin Endocrinol Metab 2003;88:141–7.
- Strowitzki T, Capp E, von Eye CH. The degree of cycle irregularity correlates with the grade of endocrine and metabolic disorders in PCOS patients. Eur J Obstet Gynecol Reprod Biol 2010;149:178–81.
- Alsamarai S, Adams JM, Murphy MK, Post MD, Hayden DL, Hall JE, et al. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab 2009;94: 4961–70.
- Burgers JA, Fong SL, Louwers YV, Valkenburg O, de Jong FH, Fauser BC, et al. Oligoovulatory and anovulatory cycles in women with polycystic ovary syndrome (PCOS): what’s the difference? J Clin Endocrinol Metab 2010;95:E485–9.
- Di FG, Mansueto P, Longo RA, Rini G, Carmina E. Influence of sociocultural factors on the ovulatory status of polycystic ovary syndrome. Fertil Steril 2009;91:1853–6.
- Koivunen R, Pouta A, Franks S, Martikainen H, Sovio U, Hartikainen AL, et al. Fecundability and spontaneous abortions in women with self-reported oligo-amenorrhea and/or hirsutism: Northern Finland Birth Cohort 1966 Study. Hum Reprod 2008;23:2134–9.
- Imani B, Eijkemans MJ, te Velde ER, Habbema JD, Fauser BC. A nomogram to predict the probability of live birth after clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. Fertil Steril 2002;77:91–7.
- Nyboe AA, Balen AH, Platteau P, Pettersson G, Arce JC. Prestimulation parameters predicting live birth in anovulatory WHO Group II patients undergoing ovulation induction with gonadotrophins. Hum Reprod 2010;25: 1988–95.
- Jones GL, Hall JM, Balen AH, Ledger WL. Health-related quality of life measurement in women with polycystic ovary syndrome: a systematic review. Hum Reprod Update 2008;14:15–25.
- Hahn S, Janssen OE, Tan S, Pleger K, Mann K, Schedlowski M, et al. Clinical and psychological correlates of quality-of-life in polycystic ovary syndrome. Eur J Endocrinol 2005;153:853–60.
- de Niet JE, de Koning CM, Pastoor H, Duivenvoorden HJ, Valkenburg O, Ramakers MJ, et al. Psychological well-being and sexarche in women with polycystic ovary syndrome. Hum Reprod 2010;25:1497–503.
- Solomon CG, Hu FB, Dunaif A, Rich-Edwards JE, Stampfer MJ, Willett WC, et al. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab 2002;87:2013–7.
- Taponen S, Martikainen H, Jarvelin MR, Sovio U, Laitinen J, Pouta A, et al. Metabolic cardiovascular disease risk factors in women with self-reported symptoms of oligomenorrhea and/or hirsutism: Northern Finland Birth Cohort 1966 Study. J Clin Endocrinol Metab 2004;89:2114–8.
- Elting MW, Korsen TJ, Schoemaker J. Obesity, rather than menstrual cycle pattern or follicle cohort size, determines hyperinsulinaemia, dyslipidaemia and hypertension in ageing women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2001;55:767–76.
- Schindler AE. Progestogen deficiency and endometrial cancer risk. Maturitas 2009;62:334–7.
- Cheung AP. Ultrasound and menstrual history in predicting endometrial hyperplasia in polycystic ovary syndrome. Obstet Gynecol 2001;98: 325–31.
- Vrbikova J, Cibula D. Combined oral contraceptives in the treatment of polycystic ovary syndrome. Hum Reprod Update 2005;11:277–91.
- Schwingl PJ, Ory HW, Visness CM. Estimates of the risk of cardiovascular death attributable to low-dose oral contraceptives in the United States. Am J Obstet Gynecol 1999;180:241–9.
- Merz CN, Johnson BD, Berga S, Braunstein G, Reis SE, Bittner V. Past oral contraceptive use and angiographic coronary artery disease in postmenopausal women: data from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation. Fertil Steril 2006;85:1425–31.
- Yildiz BO. Oral contraceptives in polycystic ovary syndrome: risk-benefit assessment. Semin Reprod Med 2008;26:111–20.
- Costello MF, Shrestha B, Eden J, Johnson NP, Sjoblom P. Metformin versus oral contraceptive pill in polycystic ovary syndrome: a Cochrane review. Hum Reprod 2007;22:1200–9.
- Nader S, Riad-Gabriel MG, Saad MF. The effect of a desogestrel-containing oral contraceptive on glucose tolerance and leptin concentrations in hyperandrogenic women. J Clin Endocrinol Metab 1997;82:3074–7.
- Morin-Papunen LC, Vauhkonen I, Koivunen RM, Ruokonen A, Martikainen HK, Tapanainen JS. Endocrine and metabolic effects of metformin versus ethinyl estradiol–cyproterone acetate in obese women with polycystic ovary syndrome: a randomized study. J Clin Endocrinol Metab 2000;85:3161–8.
- Falsetti L, Pasinetti E. Effects of long-term administration of an oral contraceptive containing ethinylestradiol and cyproterone acetate on lipid metabolism in women with polycystic ovary syndrome. Acta Obstet Gynecol Scand 1995;74:56–60.
- Pasquali R, Gambineri A, Anconetani B, Vicennati V, Colitta D, Caramelli E, et al. The natural history of the metabolic syndrome in young women with the polycystic ovary syndrome and the effect of long-term oestrogen-progestagen treatment. Clin Endocrinol (Oxf) 1999;50:517–27.
- Gaffield ME, Culwell KR. New recommendations on the safety of contraceptive methods for women with medical conditions. IPPF Med Bull 2011;44:1–5.
- Vrbikova J, Stanicka S, Dvorakova K, Hill M, Vondra K, Bendlova B, et al. Metabolic and endocrine effects of treatment with peroral or transdermal oestrogens in conjunction with peroral cyproterone acetate in women with polycystic ovary syndrome. Eur J Endocrinol 2004;150:215–23.
- Battaglia C, Mancini F, Fabbri R, Persico N, Busacchi P, Facchinetti F, et al. Polycystic ovary syndrome and cardiovascular risk in young patients treated with drospirenone-ethinylestradiol or contraceptive vaginal ring. A prospective, randomized, pilot study. Fertil Steril 2010;94:1417–25.
- Himelein MJ, Thatcher SS. Polycystic ovary syndrome and mental health: a review. Obstet Gynecol Surv 2006;61:723–32.
- Moran LJ, Brinkworth G, Noakes M, Norman RJ. Effects of lifestyle modification in polycystic ovarian syndrome. Reprod Biomed Online 2006;12: 569–78.
- Thessaloniki ESHRE/ASRM sponsored PCOS consensus workshop group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril 2008;89:505–22.
- Thessaloniki ESHRE/ASRM sponsored PCOS consensus workshop group. Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod 2008;23:462–77.
- Lim SS, Noakes M, Norman RJ. Dietary effects on fertility treatment and pregnancy outcomes. Curr Opin Endocrinol Diabetes Obes 2007;14: 465–9.
- Snyder BS. The lived experience of women diagnosed with polycystic ovary syndrome. J Obstet Gynecol Neonatal Nurs 2006;35:385–92.
- Kitzinger C, Willmott J. ‘‘The thief of womanhood’’: women’s experience of polycystic ovarian syndrome. Soc Sci Med 2002;54:349–61.
- Drosdzol A, Skrzypulec V, Mazur B, Pawlinska-Chmara R. Quality of life and marital sexual satisfaction in women with polycystic ovary syndrome. Folia Histochem Cytobiol 2007;45(Suppl 1):S93–7.
- Benson S, Janssen OE, Hahn S, Tan S, Dietz T, Mann K, et al. Obesity, depression, and chronic low-grade inflammation in women with polycystic ovary syndrome. Brain Behav Immun 2008;22:177–84.
- Tan S, Hahn S, Benson S, Janssen OE, Dietz T, Kimmig R, et al. Psychological implications of infertility in women with polycystic ovary syndrome. Hum Reprod 2008;23:2064–71.
- Barnard L, Ferriday D, Guenther N, Strauss B, Balen AH, Dye L. Quality of life and psychological well being in polycystic ovary syndrome. Hum Reprod 2007;22:2279–86.
- Benson S, Arck PC, Tan S, Hahn S, Mann K, Rifaie N, et al. Disturbed stress responses in women with polycystic ovary syndrome. Psychoneuroendocrinology 2009;34:727–35.
- Deeks AA, Gibson-Helm ME, Teede HJ. Anxiety and depression in polycystic ovary syndrome: a comprehensive investigation. Fertil Steril 2010;93: 2421–3.
- Kerchner A, Lester W, Stuart SP, Dokras A. Risk of depression and other mental health disorders in women with polycystic ovary syndrome: a longitudinal study. Fertil Steril 2009;91:207–12.
- Moran L, Gibson-Helm M, Teede H, Deeks A. Polycystic ovary syndrome: a biopsychosocial understanding in young women to improve knowledge and treatment options. J Psychosom Obstet Gynaecol 2010;31:24–31.
- Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2010;16:347–63.
- Moran LJ, Noakes M, Clifton PM, Norman RJ. The effect of modifying dietary protein and carbohydrate in weight loss on arterial compliance and postprandial lipidemia in overweight women with polycystic ovary syndrome. Fertil Steril 2010;94:2451–4.
- Mansson M, Holte J, Landin-Wilhelmsen K, Dahlgren E, Johansson A, Landen M. Women with polycystic ovary syndrome are often depressed or anxious—a case control study. Psychoneuroendocrinology 2008;33: 1132–8.
- Jedel E, Waern M, Gustafson D, Landen M, Eriksson E, Holm G, et al. Anxiety and depression symptoms in women with polycystic ovary syndrome compared with controls matched for body mass index. Hum Reprod 2010;25:450–6.
- Ching HL, Burke V, Stuckey BG. Quality of life and psychological morbidity in women with polycystic ovary syndrome: body mass index, age and the provision of patient information are significant modifiers. Clin Endocrinol (Oxf) 2007;66:373–9.
- Harris-Glocker M, Davidson K, Kochman L, Guzick D, Hoeger K. Improvement in quality-of-life questionnaire measures in obese adolescent females with polycystic ovary syndrome treated with lifestyle changes and oral contraceptives, with or without metformin. Fertil Steril 2010;93:1016–9.
- Laggari V, Diareme S, Christogiorgos S, Deligeoroglou E, Christopoulos P, Tsiantis J, et al. Anxiety and depression in adolescents with polycystic ovary syndrome and Mayer-Rokitansky-Kuster-Hauser syndrome. J Psychosom Obstet Gynaecol 2009;30:83–8.
- Rofey DL, Szigethy EM, Noll RB, Dahl RE, Lobst E, Arslanian SA. Cognitivebehavioral therapy for physical and emotional disturbances in adolescents with polycystic ovary syndrome: a pilot study. J Pediatr Psychol 2009;34: 156–63.
- Adali E, Yildizhan R, Kurdoglu M, Kolusari A, Edirne T, Sahin HG, et al. The relationship between clinico-biochemical characteristics and psychiatric distress in young women with polycystic ovary syndrome. J Int Med Res 2008;36:1188–96.
- Sundararaman PG, Shweta, Sridhar GR. Psychosocial aspects of women with polycystic ovary syndrome from south India. J Assoc Physicians India 2008;56:945–8.
- Hollinrake E, Abreu A, Maifeld M, Van Voorhis BJ, Dokras A. Increased risk of depressive disorders in women with polycystic ovary syndrome. Fertil Steril 2007;87:1369–76.
- Battaglia C, Nappi RE, Mancini F, Cianciosi A, Persico N, Busacchi P, et al. PCOS, sexuality, and clitoral vascularisation: a pilot study. J Sex Med 2008;5:2886–94.
- Anger JT, Brown AJ, Amundsen CL. Sexual dysfunction in women with polycystic ovary syndrome: the effects of testosterone, obesity, and depression. Female Pelvic Med Reconstr Surg 2007;13:119–24.
- Trent ME, Rich M, Austin SB, Gordon CM. Fertility concerns and sexual behavior in adolescent girls with polycystic ovary syndrome: implications for quality of life. J Pediatr Adolesc Gynecol 2003;16:33–7.
- Thomson RL, Buckley JD, Lim SS, Noakes M, Clifton PM, Norman RJ, et al. Lifestyle management improves quality of life and depression in overweight and obese women with polycystic ovary syndrome. Fertil Steril 2010;94:1812–6.
- Palomba S, Giallauria F, Falbo A, Russo T, Oppedisano R, Tolino A, et al. Structured exercise training programme versus hypocaloric hyperproteic diet in obese polycystic ovary syndrome patients with anovulatory infertility: a 24-week pilot study. Hum Reprod 2008;23:642–50.
- Lipton MG, Sherr L, Elford J, Rustin MH, Clayton WJ. Women living with facial hair: the psychological and behavioral burden. J Psychosom Res 2006;61:161–8.
- Percy CA, Gibbs T, Potter L, Boardman S. Nurse-led peer support group: experiences of women with polycystic ovary syndrome. J Adv Nurs 2009;65: 2046–55.
- Benson S, Hahn S, Tan S, Mann K, Janssen OE, Schedlowski M, et al. Prevalence and implications of anxiety in polycystic ovary syndrome: results of an internet-based survey in Germany. Hum Reprod 2009;24:1446–51.
- Goverde A, Westervald E, Verhulst S, Fauser B. Polycystic ovary syndrome as a developmental disorder. Exp Rev Obstret Gynecol 2008;3:775–87.
- Malik-Aslam A, Reaney MD, Speight J. The suitability of polycystic ovary syndrome-specific questionnaires for measuring the impact of PCOS on quality of life in clinical trials. Value Health 2010;13:440–6.
- Boyle P, Vainio H, Smith R, Benamouzig R, Lee WC, Segnan N, et al. Workgroup I: criteria for screening. UICC International Workshop on Facilitating Screening for Colorectal Cancer, Oslo, Norway (29 and 30 June 2002). Ann Oncol 2005;16:25–30.
- Boivin J. A review of psychosocial interventions in infertility. Soc Sci Med 2003;57:2325–41.
- Dumesic DA, Padmanabhan V, Abbott DH. Polycystic ovary syndrome and oocyte developmental competence. Obstet Gynecol Surv 2008;63:39–48.
- Weghofer A, Munne S, Chen S, Barad D, Gleicher N. Lack of association between polycystic ovary syndrome and embryonic aneuploidy. Fertil Steril 2007;88:900–5.
- Rice S, Christoforidis N, Gadd C, Nikolaou D, Seyani L, Donaldson A, et al. Impaired insulin-dependent glucose metabolism in granulosa-lutein cells from anovulatory women with polycystic ovaries. Hum Reprod 2005;20: 373–81.
- Skov V, Glintborg D, Knudsen S, Jensen T, Kruse TA, Tan Q, et al. Reduced expression of nuclear-encoded genes involved in mitochondrial oxidative metabolism in skeletal muscle of insulin-resistant women with polycystic ovary syndrome. Diabetes 2007;56:2349–55.
- Wood JR, Dumesic DA, Abbott DH, Strauss JF III. Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab 2007;92:705–13.
- Harris SE, Maruthini D, Tang T, Balen AH, Picton HM. Metabolism and karyotype analysis of oocytes from patients with polycystic ovary syndrome. Hum Reprod 2010;25:2305–15.
- Hickey TE, Legro RS, Norman RJ. Epigenetic modification of the X chromosome influences susceptibility to polycystic ovary syndrome. J Clin Endocrinol Metab 2006;91:2789–91.
- Li Z, Huang H. Epigenetic abnormality: a possible mechanism underlying the fetal origin of polycystic ovary syndrome. Med Hypotheses 2008;70: 638–42.
- Homburg R. Pregnancy complications in PCOS. Best Pract Res Clin Endocrinol Metab 2006;20:281–92.
- Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 2007;356:551–66.
- Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev 2010;1:CD003053.
- Vanky E, Stridsklev S, Heimstad R, Romundstad P, Skogoy K, Kleggetveit O, et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: a randomized, controlled multicenter study. J Clin Endocrinol Metab 2010;95:E448–55.
- Giudice LC. Endometrium in PCOS: implantation and predisposition to endocrine CA. Best Pract Res Clin Endocrinol Metab 2006;20:235–44.
- Veltman-Verhulst SM, van Haeften TW, Eijkemans MJ, de Valk HW, Fauser BC, Goverde AJ. Sex hormone-binding globulin concentrations before conception as a predictor for gestational diabetes in women with polycystic ovary syndrome. Hum Reprod 2010;25:3123–8.
- Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update 2006;12:673–83.
- Palomba S, Falbo A, Russo T, Battista L, Tolino A, Orio F, et al. Uterine blood flow in pregnant patients with polycystic ovary syndrome: relationships with clinical outcomes. BJOG 2010;117:711–21.
- Wax JR. Risks and management of obesity in pregnancy: current controversies. Curr Opin Obstet Gynecol 2009;21:117–23.
- Nelson SM, Matthews P, Poston L. Maternal metabolism and obesity: modifiable determinants of pregnancy outcome. Hum Reprod Update 2010;16: 255–75.
- Palomba S, Falbo A, Orio F Jr, Zullo F. Effect of preconceptional metformin on abortion risk in polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril 2009;92:1646–58.
- Vanky E, Salvesen KA, Heimstad R, Fougner KJ, Romundstad P, Carlsen SM. Metformin reduces pregnancy complications without affecting androgen levels in pregnant polycystic ovary syndrome women: results of a randomized study. Hum Reprod 2004;19:1734–40.
- Dunaif A, Sorbara L, Delson R, Green G. Ethnicity and polycystic ovary syndrome are associated with independent and additive decreases in insulin action in Caribbean-Hispanic women. Diabetes 1993;42:1462–8.
- Kauffman RP, Baker VM, Dimarino P, Gimpel T, Castracane VD. Polycystic ovarian syndrome and insulin resistance in white and Mexican American women: a comparison of two distinct populations. Am J Obstet Gynecol 2002;187:1362–9.
- Kauffman RP, Baker VM, Dimarino P, Castracane VD. Hyperinsulinemia and circulating dehydroepiandrosterone sulfate in white and Mexican American women with polycystic ovary syndrome. Fertil Steril 2006;85: 1010–6.
- Goodarzi MO, Quinones MJ, Azziz R, Rotter JI, Hsueh WA, Yang H. Polycystic ovary syndrome in Mexican-Americans: prevalence and association with the severity of insulin resistance. Fertil Steril 2005;84:766–9.
- Carmina E, Koyama T, Chang L, Stanczyk FZ, Lobo RA. Does ethnicity influence the prevalence of adrenal hyperandrogenism and insulin resistance in polycystic ovary syndrome? Am J Obstet Gynecol 1992;167:1807–12.
- Chen X, Yang D, Mo Y, Li L, Chen Y, Huang Y. Prevalence of polycystic ovary syndrome in unselected women from southern China. Eur J Obstet Gynecol Reprod Biol 2008;139:59–64.
- Ng EHY, Ho PC. Polycystic ovary syndrome in Asian women. Semin Reprod Med 2008;26:14–21.
- Lin TC, Yen JM, Gong KB, Kuo TC, Ku DC, Liang SF, et al. Abnormal glucose tolerance and insulin resistance in polycystic ovary syndrome amongst the Taiwanese population—not correlated with insulin receptor substrate-1 Gly972Arg/Ala513Pro polymorphism. BMC Med Genet 2006;7:36.
- Li L, Yang D, Chen X, Chen Y, Feng S, Wang L. Clinical and metabolic features of polycystic ovary syndrome. Int J Gynaecol Obstet 2007;97:129–34.
- Norman RJ, Mahabeer S, Masters S. Ethnic differences in insulin and glucose response to glucose between white and Indian women with polycystic ovary syndrome. Fertil Steril 1995;63:58–62.
- Rodin DA, Bano G, Bland JM, Taylor K, Nussey SS. Polycystic ovaries and associated metabolic abnormalities in Indian subcontinent Asian women. Clin Endocrinol (Oxf) 1998;49:91–9.
- Wijeyaratne CN, Balen AH, Barth JH, Belchetz PE. Clinical manifestations and insulin resistance (IR) in polycystic ovary syndrome (PCOS) among South Asians and Caucasians: is there a difference? Clin Endocrinol (Oxf) 2002;57:343–50.
- Wijeyaratne CN, Nirantharakumar K, Balen AH, Barth JH, Sheriff R, Belchetz PE. Plasma homocysteine in polycystic ovary syndrome: does it correlate with insulin resistance and ethnicity? Clin Endocrinol (Oxf) 2004;60:560–7.
- Sundararaman PG, Manomani R, Sridhar GR, Sridhar V, Sundaravalli A, Umachander M. Risk of atherosclerosis in women with polycystic ovary syndrome: a study from South India. Metab Syndr Relat Disord 2003;1:271–5.
- Kalra P, Bansal B, Nag P, Singh JK, Gupta RK, Kumar S, et al. Abdominal fat distribution and insulin resistance in Indian women with polycystic ovarian syndrome. Fertil Steril 2009;91:1437–40.
- Charnvises K, Weerakiet S, Tingthanatikul Y, Wansumrith S, Chanprasertyothin S, Rojanasakul A. Acanthosis nigricans: clinical predictor of abnormal glucose tolerance in Asian women with polycystic ovary syndrome. Gynecol Endocrinol 2005;21:161–4.
- Weerakiet S, Bunnag P, Phakdeekitcharoen B, Wansumrith S, Chanprasertyothin S, Jultanmas R, et al. Prevalence of the metabolic syndrome in Asian women with polycystic ovary syndrome: using the International Diabetes Federation criteria. Gynecol Endocrinol 2007;23:153–60.
- Lo JC, Feigenbaum SL, Yang J, Pressman AR, Selby JV, Go AS. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab 2006;91:1357–63.
- Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2006;91:48–53.
- Legro RS, Myers ER, Barnhart HX, Carson SA, Diamond MP, Carr BR, et al. The Pregnancy in Polycystic Ovary Syndrome study: baseline characteristics of the randomized cohort including racial effects. Fertil Steril 2006;86: 914–33.
- Welt CK, Arason G, Gudmundsson JA, Adams J, Palsdottir H, Gudlaugsdottir G, et al. Defining constant versus variable phenotypic features of women with polycystic ovary syndrome using different ethnic groups and populations. J Clin Endocrinol Metab 2006;91:4361–8.
- Welt CK, Gudmundsson JA, Arason G, Adams J, Palsdottir H, Gudlaugsdottir G, et al. Characterizing discrete subsets of polycystic ovary syndrome as defined by the Rotterdam criteria: the impact of weight on VOL. 97 NO. 1 / JANUARY 2012 38.e21 Fertility and Sterility® phenotype and metabolic features. J Clin Endocrinol Metab 2006;91: 4842–8.
- Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab 1999;84:4006–11.
- Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab 2000;85:2434–8.
- Vural B, Caliskan E, Turkoz E, Kilic T, Demirci A. Evaluation of metabolic syndrome frequency and premature carotid atherosclerosis in young women with polycystic ovary syndrome. Hum Reprod 2005;20:2409–13.
- Williamson K, Gunn AJ, Johnson N, Milsom SR. The impact of ethnicity on the presentation of polycystic ovarian syndrome. Aust NZ J Obstet Gynaecol 2001;41:202–6.
- Al-Fozan H, Al-Futaisi A, Morris D, Tulandi T. Insulin responses to the oral glucose tolerance test in women of different ethnicity with polycystic ovary syndrome. J Obstet Gynaecol Can 2005;27:33–7.
- Schmid J, Kirchengast S, Vytiska-Binstorfer E, Huber J. Psychosocial and sociocultural aspects of infertility—a comparison between Austrian women and immigrant women. Anthropol Anz 2004;62:301–9.
- Najem F, Elmehdawi R, Swalem A. Clinical and biochemical characteristics of polycystic ovary syndrome in Benghazi-Libya: a retrospective study. Libyan J Med 2008;3:71–4.
- Al-Ruhaily AD, Malabu UH, Sulimani RA. Hirsutism in Saudi females of reproductive age: a hospital-based study. Ann Saudi Med 2008;28: 28–32.
- Wijeyaratne CN, Seneviratne RA, Dahanayake S, Kumarapeli V, Palipane E, Kuruppu N, et al. Phenotype and metabolic profile of South Asian women with polycystic ovary syndrome (PCOS): results of a large database from a specialist endocrine clinic. Hum Reprod 2011;26:202–13.
- Iwasa T, Matsuzaki T, Minakuchi M, Tanaka N, Shimizu F, Hirata Y, et al. Diagnostic performance of serum total testosterone for Japanese patients with polycystic ovary syndrome. Endocr J 2007;54:233–8.
- Vrbikova J, Vondra K, Cibula D, Dvorakova K, Stanicka S, Sramkova D, et al. Metabolic syndrome in young Czech women with polycystic ovary syndrome. Hum Reprod 2005;20:3328–32.
- Wijeyeratne CN, Kumarapeli V, Seneviratne RD, Antonypillai CN, Seneviratne RD, Chaminda GJ, et al. Ethnic variations in the expression of polycystic ovary syndrome. In: Balen AH, Franks S, Homburg R, Kehoe S, editors. Current management of polycystic ovary syndrome. London: RCOG Press; 2010:25–46.
- Kumarapeli V, Seneviratne RA, Wijeyaratne C. Health-related quality of life and psychological distress in polycystic ovary syndrome: a hidden facet in South Asian women. BJOG 2011;118:319–28.
- Fulghesu A, Magnini R, Portoghese E, Angioni S, Minerba L, Melis GB. Obesity-related lipid profile and altered insulin incretion in adolescents with polycystic ovary syndrome. J Adolesc Health 2010;46:474–81.
- Al-Azemi M, Omu FE, Omu AE. The effect of obesity on the outcome of infertility management in women with polycystic ovary syndrome. Arch Gynecol Obstet 2004;270:205–10.
- Glueck CJ, Dharashivkar S, Wang P, Zhu B, Gartside PS, Tracy T, et al. Obesity and extreme obesity, manifest by ages 20–24 years, continuing through 32–41 years in women, should alert physicians to the diagnostic likelihood of polycystic ovary syndrome as a reversible underlying endocrinopathy. Eur J Obstet Gynecol Reprod Biol 2005;122:206–12.
- Trent M, Austin SB, Rich M, Gordon CM. Overweight status of adolescent girls with polycystic ovary syndrome: body mass index as mediator of quality of life. Ambul Pediatr 2005;5:107–11.
- Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab 2006;91:492–7.
- Farid-ur-Rehman SI, Hayat Z, Niazi NA. Etiology of hirsutism: is there a correlation between menstrual irregularity, body mass index and severity of hirsutism with the cause? J Pakistan Assoc Dermatol 2010;20:4–9.
- Lord J, Thomas R, Fox B, Acharya U, Wilkin T. The central issue? Visceral fat mass is a good marker of insulin resistance and metabolic disturbance in women with polycystic ovary syndrome. BJOG 2006;113:1203–9.
- Marciniak A, Nawrocka-Rutkowska J, Brodowska A, Sienkiewicz R, Szydlowska I, Starczewski A. Leptin concentrations in patients with polycystic ovary syndrome before and after metformin treatment depending on insulin resistance, body mass index and androgen concentrations—introductory report. Folia Histochem Cytobiol 2009;47:323–8.
- Chen X, Ni R, Mo Y, Li L, Yang D. Appropriate BMI levels for PCOS patients in Southern China. Hum Reprod 2010;25:1295–302.
- Zhao X, Zhong J, Mo Y, Chen X, Chen Y, Yang D. Association of biochemical hyperandrogenism with type 2 diabetes and obesity in Chinese women with polycystic ovary syndrome. Int J Gynaecol Obstet 2010;108:148–51.
- Holte J, Bergh T, Berne C, Lithell H. Serum lipoprotein lipid profile in women with the polycystic ovary syndrome: relation to anthropometric, endocrine and metabolic variables. Clin Endocrinol (Oxf) 1994;41:463–71.
- Kirchengast S, Huber J. Body composition characteristics and body fat distribution in lean women with polycystic ovary syndrome. Hum Reprod 2001;16:1255–60.
- Yucel A, Noyan V, Sagsoz N. The association of serum androgens and insulin resistance with fat distribution in polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2006;126:81–6.
- Pasquali R, Casimirri F, Venturoli S, Antonio M, Morselli L, Reho S, et al. Body fat distribution has weight-independent effects on clinical, hormonal, and metabolic features of women with polycystic ovary syndrome. Metabolism 1994;43:706–13.
- Douchi T, Ijuin H, Nakamura S, Oki T, Yamamoto S, Nagata Y. Body fat distribution in women with polycystic ovary syndrome. Obstet Gynecol 1995;86: 516–9.
- Franks S, Kiddy D, Sharp P, Singh A, Reed M, Seppala M, et al. Obesity and polycystic ovary syndrome. Ann NY Acad Sci 1991;626:201–6.
- Hamilton-Fairley D, Kiddy D, Watson H, Paterson C, Franks S. Association of moderate obesity with a poor pregnancy outcome in women with polycystic ovary syndrome treated with low dose gonadotrophin. Br J Obstet Gynaecol 1992;99:128–31.
- Cibula D, Hill M, Fanta M, Sindelka G, Zivny J. Does obesity diminish the positive effect of oral contraceptive treatment on hyperandrogenism in women with polycystic ovarian syndrome? Hum Reprod 2001;16:940–4.
- Tamimi W, Siddiqui IA, Tamim H, AlEisa N, Adham M. Effect of body mass index on clinical manifestations in patients with polycystic ovary syndrome. Int J Gynaecol Obstet 2009;107:54–7.
- Acien P, Quereda F, Matallin P, Villarroya E, Lopez-Fernandez JA, Acien M, et al. Insulin, androgens, and obesity in women with and without polycystic ovary syndrome: a heterogeneous group of disorders. Fertil Steril 1999;72: 32–40.
- Piouka A, Farmakiotis D, Katsikis I, Macut D, Gerou S, Panidis D. Antimullerian hormone levels reflect severity of PCOS but are negatively influenced by obesity: relationship with increased luteinizing hormone levels. Am J Physiol Endocrinol Metab 2009;296:E238–43.
- Hahn S, Backhaus M, Broecker-Preuss M, Tan S, Dietz T, Kimmig R, et al. Retinol-binding protein 4 levels are elevated in polycystic ovary syndrome women with obesity and impaired glucose metabolism. Eur J Endocrinol 2007;157:201–7.
- Godoy-Matos AF, Vaisman F, Pedrosa AP, Farias ML, Mendonca LM, Pinheiro MF. Central-to-peripheral fat ratio, but not peripheral body fat, is related to insulin resistance and androgen markers in polycystic ovary syndrome. Gynecol Endocrinol 2009:1–6.
- Douchi T, Yoshimitsu N, Nagata Y. Relationships among serum testosterone levels, body fat and muscle mass distribution in women with polycystic ovary syndrome. Endocr J 2001;48:685–9.
- Al-Bayatti AA. Insulin resistance and upper-body obesity in polycystic ovary syndrome. Middle East Fertil Soc J 2006;11:202–9.
- Carmina E, Bucchieri S, Esposito A, Del PA, Mansueto P, Orio F, et al. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. J Clin Endocrinol Metab 2007;92:2500–5.
- Cascella T, Palomba S, De Sio I, Manguso F, Giallauria F, De Simone B, et al. Visceral fat is associated with cardiovascular risk in women with polycystic ovary syndrome. Hum Reprod 2008;23:153–9.
- Kiddy DS, Hamilton-Fairley D, Bush A, Short F, Anyaoku V, Reed MJ, et al. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 1992;36:105–11.
- Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod 1998;13:1502–5.
- Moran LJ, Hutchison SK, Norman RJ, Teede HJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2011;2: CD007506.
- Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 1997;18:774–800.
- Dunaif A, Graf M, Mandeli J, Laumas V, Dobrjansky A. Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J Clin Endocrinol Metab 1987;65:499–507.
- Robinson S, Kiddy D, Gelding SV, Willis D, Niththyananthan R, Bush A, et al. The relationship of insulin insensitivity to menstrual pattern in women with hyperandrogenism and polycystic ovaries. Clin Endocrinol (Oxf) 1993;39: 351–5.
- Goverde AJ, van Koert AJ, Eijkemans MJ, Knauff EA, Westerveld HE, Fauser BC, et al. Indicators for metabolic disturbances in anovulatory women with polycystic ovary syndrome diagnosed according to the Rotterdam consensus criteria. Hum Reprod 2009;24:710–7.
- Moran L, Teede H. Metabolic features of the reproductive phenotypes of polycystic ovary syndrome. Hum Reprod Update 2009;15:477–88.
- Legro RS, Chiu P, Kunselman AR, Bentley CM, Dodson WC, Dunaif A. Polycystic ovaries are common in women with hyperandrogenic chronic anovulation but do not predict metabolic or reproductive phenotype. J Clin Endocrinol Metab 2005;90:2571–9.
- Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab 2005;90:3236–42.
- Jovanovic VP, Carmina E, Lobo RA. Not all women diagnosed with PCOS share the same cardiovascular risk profiles. Fertil Steril 2010;94: 826–32.
- Barber TM, Bennett AJ, Gloyn AL, Groves CJ, Sovio U, Ruokonen A, et al. Relationship between E23K (an established type II diabetes-susceptibility variant within KCNJ11), polycystic ovary syndrome and androgen levels. Eur J Hum Genet 2007;15:679–84.
- Sam S, Dunaif A. Polycystic ovary syndrome: syndrome XX? Trends Endocrinol Metab 2003;14:365–70.
- Ehrmann DA, Sturis J, Byrne MM, Karrison T, Rosenfield RL, Polonsky KS. Insulin secretory defects in polycystic ovary syndrome: relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. J Clin Invest 1995;96:520–7.
- Biyasheva A, Legro RS, Dunaif A, Urbanek M. Evidence for association between polycystic ovary syndrome (PCOS) and TCF7L2 and glucose intolerance in women with PCOS and TCF7L2. J Clin Endocrinol Metab 2009;94: 2617–25.
- Bergman RN. Orchestration of glucose homeostasis: from a small acorn to the California oak. Diabetes 2007;56:1489–501.
- Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr 1956;4:20–34.
- Kirschner MA, Samojlik E, Drejka M, Szmal E, Schneider G, Ertel N. Androgen-estrogen metabolism in women with upper body versus lower body obesity. J Clin Endocrinol Metab 1990;70:473–9.
- Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril 2006;85:1319–40.
- Dumesic DA, Abbott DH, Eisner JR, Herrmann RR, Reed JE, Welch TJ, et al. Pituitary desensitization to gonadotropin-releasing hormone increases abdominal adiposity in hyperandrogenic anovulatory women. Fertil Steril 1998;70:94–101.
- Barber TM, Bennett AJ, Groves CJ, Sovio U, Ruokonen A, Martikainen H, et al. Association of variants in the fat mass and obesity associated (FTO) gene with polycystic ovary syndrome. Diabetologia 2008;51:1153–8.
- Barber TM, Golding SJ, Alvey C, Wass JA, Karpe F, Franks S, et al. Global adiposity rather than abnormal regional fat distribution characterizes women with polycystic ovary syndrome. J Clin Endocrinol Metab 2008;93:999–1004.
- Faulds G, Ryden M, Ek I, Wahrenberg H, Arner P. Mechanisms behind lipolytic catecholamine resistance of subcutaneous fat cells in the polycystic ovarian syndrome. J Clin Endocrinol Metab 2003;88:2269–73.
- Ek I, Arner P, Ryden M, Holm C, Thorne A, Hoffstedt J, et al. A unique defect in the regulation of visceral fat cell lipolysis in the polycystic ovary syndrome as an early link to insulin resistance. Diabetes 2002;51:484–92.
- Legro RS, Driscoll D, Strauss JF III, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci USA 1998;95:14956–60.
- Baillargeon JP, Nestler JE. Commentary: polycystic ovary syndrome: a syndrome of ovarian hypersensitivity to insulin? J Clin Endocrinol Metab 2006;91:22–4.
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: executive summary. Crit Pathw Cardiol 2005;4:198–203.
- Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2005;90:1929–35.
- Korhonen S, Hippelainen M, Vanhala M, Heinonen S, Niskanen L. The androgenic sex hormone profile is an essential feature of metabolic syndrome in premenopausal women: a controlled community-based study. Fertil Steril 2003;79:1327–34.
- Gambineri A, Patton L, Vaccina A, Cacciari M, Morselli-Labate AM, Cavazza C, et al. Treatment with flutamide, metformin, and their combination added to a hypocaloric diet in overweight-obese women with polycystic ovary syndrome: a randomized, 12-month, placebo-controlled study. J Clin Endocrinol Metab 2006;91:3970–80.
- Robinson S, Chan SP, Spacey S, Anyaoku V, Johnston DG, Franks S. Postprandial thermogenesis is reduced in polycystic ovary syndrome and is associated with increased insulin resistance. Clin Endocrinol (Oxf) 1992;36:537–43.
- Venkatesan AM, Dunaif A, Corbould A. Insulin resistance in polycystic ovary syndrome: progress and paradoxes. Recent Prog Horm Res 2001;56:295–308.
- Barber TM, Wass JA, McCarthy MI, Franks S. Metabolic characteristics of women with polycystic ovaries and oligo-amenorrhoea but normal androgen levels: implications for the management of polycystic ovary syndrome. Clin Endocrinol (Oxf) 2007;66:513–7.
- Dewailly D, Catteau-Jonard S, Reyss AC, Leroy M, Pigny P. Oligoanovulation with polycystic ovaries but not overt hyperandrogenism. J Clin Endocrinol Metab 2006;91:3922–7.
- Robinson S, Henderson AD, Gelding SV, Kiddy D, Niththyananthan R, Bush A, et al. Dyslipidaemia is associated with insulin resistance in women with polycystic ovaries. Clin Endocrinol (Oxf) 1996;44:277–84.
- Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care 1999;22:141–6.
- Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 1999;84:165–9.
- Solomon CG, Hu FB, Dunaif A, Rich-Edwards J, Willett WC, Hunter DJ, et al. Long or highly irregular menstrual cycles as a marker for risk of type 2 diabetes mellitus. JAMA 2001;286:2421–6.
- Wild S, Pierpoint T, Jacobs H, McKeigue P. Long-term consequences of polycystic ovary syndrome: results of a 31 year follow-up study. Hum Fertil (Camb) 2000;3:101–5. VOL. 97 NO. 1 / JANUARY 2012 38.e23 Fertility and Sterility®
- Wild S, Pierpoint T, McKeigue P, Jacobs H. Cardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: a retrospective cohort study. Clin Endocrinol (Oxf) 2000;52:595–600.
- Norman RJ, Masters L, Milner CR, Wang JX, Davies MJ. Relative risk of conversion from normoglycaemia to impaired glucose tolerance or non-insulin dependent diabetes mellitus in polycystic ovarian syndrome. Hum Reprod 2001;16:1995–8.
- Legro RS, Bentley-Lewis R, Driscoll D, Wang SC, Dunaif A. Insulin resistance in the sisters of women with polycystic ovary syndrome: association with hyperandrogenemia rather than menstrual irregularity. J Clin Endocrinol Metab 2002;87:2128–33.
- Franks S, Webber LJ, Goh M, Valentine A, White DM, Conway GS, et al. Ovarian morphology is a marker of heritable biochemical traits in sisters with polycystic ovaries. J Clin Endocrinol Metab 2008;93:3396–402.
- Escobar-Morreale HF, Botella-Carretero JI, Alvarez-Blasco F, Sancho J, San Millan JL. The polycystic ovary syndrome associated with morbid obesity may resolve after weight loss induced by bariatric surgery. J Clin Endocrinol Metab 2005;90:6364–9.
- Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–52.
- Douglas CC, Norris LE, Oster RA, Darnell BE, Azziz R, Gower BA. Difference in dietary intake between women with polycystic ovary syndrome and healthy controls. Fertil Steril 2006;86:411–7.
- Marsh KA, Steinbeck KS, Atkinson FS, Petocz P, Brand-Miller JC. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am J Clin Nutr 2010;92:83–92.
- Katcher HI, Kunselman AR, Dmitrovic R, Demers LM, Gnatuk CL, Kris-Etherton PM, et al. Comparison of hormonal and metabolic markers after a high-fat, Western meal versus a low-fat, high-fiber meal in women with polycystic ovary syndrome. Fertil Steril 2009;91:1175–82.
- Bishop SC, Basch S, Futterweit W. Polycystic ovary syndrome, depression, and affective disorders. Endocr Pract 2009;15:475–82.
- Dokras A, Clifton S, Futterweit W, Wild R. Increased risk for abnormal depression scores in women with polycystic ovary syndrome: a systematic review and meta-analysis. Obstet Gynecol 2011;117:145–52.
- Pirwany IR, Fleming R, Greer IA, Packard CJ, Sattar N. Lipids and lipoprotein subfractions in women with PCOS: relationship to metabolic and endocrine parameters. Clin Endocrinol (Oxf) 2001;54:447–53.
- Fogel RB, Malhotra A, Pillar G, Pittman SD, Dunaif A, White DP. Increased prevalence of obstructive sleep apnea syndrome in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 2001;86:1175–80.
- Wild RA, Grubb B, Hartz A, Van Nort JJ, Bachman W, Bartholomew M. Clinical signs of androgen excess as risk factors for coronary artery disease. Fertil Steril 1990;54:255–9.
- Wiltgen D, Spritzer PM. Variation in metabolic and cardiovascular risk in women with different polycystic ovary syndrome phenotypes. Fertil Steril 2010;94:2493–6.
- Oh JY, Sung YA, Lee HJ, Oh JY, Chung HW, Park H. Optimal waist circumference for prediction of metabolic syndrome in young Korean women with polycystic ovary syndrome. Obesity (Silver Spring) 2010;18:593–7.
- Wild RA, Rizzo M, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril 2011;95:1073–9.
- Sattar N, Gaw A, Scherbakova O, Ford I, O’Reilly DS, Haffner SM, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation 2003;108:414–9.
- Wild RA, Alaupovic P, Parker IJ. Lipid and apolipoprotein abnormalities in hirsute women. I. The association with insulin resistance. Am J Obstet Gynecol 1992;166:1191–6.
- Berneis K, Rizzo M, Lazzarini V, Fruzzetti F, Carmina E. Atherogenic lipoprotein phenotype and low-density lipoproteins size and subclasses in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2007;92:186–9.
- Samy N, Hashim M, Sayed M, Said M. Clinical significance of inflammatory markers in polycystic ovary syndrome: their relationship to insulin resistance and body mass index. Dis Markers 2009;26:163–70.
- Moran LJ, Hutchison SK, Meyer C, Zoungas S, Teede HJ. A comprehensive assessment of endothelial function in overweight women with and without polycystic ovary syndrome. Clin Sci (Lond) 2009;116:761–70.
- Soares GM, Vieira CS, Martins WP, Franceschini SA, dos Reis RM, Silva de Sa MF, et al. Increased arterial stiffness in nonobese women with polycystic ovary syndrome (PCOS) without comorbidities: one more characteristic inherent to the syndrome? Clin Endocrinol (Oxf) 2009;71:406–1.
- Krentz AJ, von MD, Barrett-Connor E. Searching for polycystic ovary syndrome in postmenopausal women: evidence of a dose-effect association with prevalent cardiovascular disease. Menopause 2007;14:284–92.
- Maturana MA, Breda V, Lhullier F, Spritzer PM. Relationship between endogenous testosterone and cardiovascular risk in early postmenopausal women. Metabolism 2008;57:961–5.
- DeUgarte CM, Bartolucci AA, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril 2005;83:1454–60.
- Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 1989;38:1165–74.
- Palmert MR, Gordon CM, Kartashov AI, Legro RS, Emans SJ, Dunaif A. Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab 2002;87:1017–23.
- Boudreaux MY, Talbott EO, Kip KE, Brooks MM, Witchel SF. Risk of T2DM and impaired fasting glucose among PCOS subjects: results of an 8-year follow-up. Curr Diab Rep 2006;6:77–83.
- Dokras A, Jagasia DH, Maifeld M, Sinkey CA, VanVoorhis BJ, Haynes WG. Obesity and insulin resistance but not hyperandrogenism mediates vascular dysfunction in women with polycystic ovary syndrome. Fertil Steril 2006;86:1702–9.
- Ketel IJ, Stehouwer CD, Henry RM, Serne EH, Hompes P, Homburg R, et al. Greater arterial stiffness in polycystic ovary syndrome (PCOS) is an obesity—but not a PCOS-associated phenomenon. J Clin Endocrinol Metab 2010;95:4566–75.
- Birdsall MA, Farquhar CM, White HD. Association between polycystic ovaries and extent of coronary artery disease in women having cardiac catheterization. Ann Intern Med 1997;126:32–5.
- Talbott EO, Guzick DS, Sutton-Tyrrell K, Hugh-Pemu KP, Zborowski JV, Remsberg KE, et al. Evidence for association between polycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women. Arterioscler Thromb Vasc Biol 2000;20:2414–21.
- Luque-Ramirez M, Mendieta-Azcona C, Alvarez-Blasco F, Escobar-Morreale HF. Androgen excess is associated with the increased carotid intima-media thickness observed in young women with polycystic ovary syndrome. Hum Reprod 2007;22:3197–203.
- Christian RC, Dumesic DA, Behrenbeck T, Oberg AL, Sheedy PF, Fitzpatrick LA. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2003;88:2562–8.
- Talbott EO, Zborowski JV, Rager JR, Boudreaux MY, Edmundowicz DA, Guzick DS. Evidence for an association between metabolic cardiovascular syndrome and coronary and aortic calcification among women with polycystic ovary syndrome. J Clin Endocrinol Metab 2004;89:5454–61.
- Talbott EO, Zborowski J, Rager J, Stragand JR. Is there an independent effect of polycystic ovary syndrome (PCOS) and menopause on the prevalence of subclinical atherosclerosis in middle aged women? Vasc Health Risk Manag 2008;4:453–62.
- Elting MW, Korsen TJ, Bezemer PD, Schoemaker J. Prevalence of diabetes mellitus, hypertension and cardiac complaints in a follow-up study of a Dutch PCOS population. Hum Reprod 2001;16:556–60.
- Cibula D, Cifkova R, Fanta M, Poledne R, Zivny J, Skibova J. Increased risk of non-insulin dependent diabetes mellitus, arterial hypertension and coronary artery disease in perimenopausal women with a history of the polycystic ovary syndrome. Hum Reprod 2000;15:785–9.
- Pierpoint T, McKeigue PM, Isaacs AJ, Wild SH, Jacobs HS. Mortality of women with polycystic ovary syndrome at long-term follow-up. J Clin Epidemiol 1998;51:581–6.
- Shaw LJ, Bairey Merz CN, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, et al. Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health—National Heart, Lung, and Blood Institute sponsored Women’s Ischemia Syndrome Evaluation. J Clin Endocrinol Metab 2008;93:1276–84.
- Azevedo GD, Duarte JM, Souza MO, Costa-E-Silva TD, Soares EM, Maranhao TM. Menstrual cycle irregularity as a marker of cardiovascular risk factors at postmenopausal years [article in Portuguese]. Arq Bras Endocrinol Metabol 2006;50:876–83.
- Chittenden BG, Fullerton G, Maheshwari A, Bhattacharya S. Polycystic ovary syndrome and the risk of gynaecological cancer: a systematic review. Reprod Biomed Online 2009;19:398–405.
- Schildkraut JM, Schwingl PJ, Bastos E, Evanoff A, Hughes C. Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet Gynecol 1996;88:554–9.
- Coulam CB, Annegers JF, Kranz JS. Chronic anovulation syndrome and associated neoplasia. Obstet Gynecol 1983;61:403–7.
- Hardiman P, Pillay OC, Atiomo W. Polycystic ovary syndrome and endometrial carcinoma. Lancet 2003;361:1810–2.
- McGowan L, Parent L, Lednar W, Norris HJ. The woman at risk for developing ovarian cancer. Gynecol Oncol 1979;7:325–44.
- Rossing MA, Daling JR, Weiss NS, Moore DE, Self SG. Ovarian tumors in a cohort of infertile women. N Engl J Med 1994;331:771–6.
- Horn-Ross PL, Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 U.S. case-control studies. VI. Nonepithelial cancers among adults. Collaborative Ovarian Cancer Group. Epidemiology 1992;3:490–5.
- Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. II. Invasive epithelial ovarian cancers in white women. Collaborative Ovarian Cancer Group. Am J Epidemiol 1992;136:1184–203.
- Harris R, Whittemore AS, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. III. Epithelial tumors of low malignant potential in white women. Collaborative Ovarian Cancer Group. Am J Epidemiol 1992;136:1204–11.
- Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, et al. Formation and early development of follicles in the polycystic ovary. Lancet 2003;362:1017–21.
- Hudecova M, Holte J, Olovsson M, Sundstrom Poromea I. Long-term follow-up of patients with polycystic ovary syndrome: reproductive outcome and ovarian reserve. Hum Reprod 2009;24:1176–83.
- Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-mullerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod 2005;20:1820–6.
- Mulders AG, Laven JS, Eijkemans MJ, de Jong FH, Themmen AP, Fauser BC. Changes in anti-mullerian hormone serum concentrations over time suggest delayed ovarian ageing in normogonadotrophic anovulatory infertility. Hum Reprod 2004;19:2036–42.
- Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab 2005;90:3847–53.
- Winters SJ, Talbott E, Guzick DS, Zborowski J, McHugh KP. Serum testosterone levels decrease in middle age in women with the polycystic ovary syndrome. Fertil Steril 2000;73:724–9.
- Imani B, Eijkemans MJ, te Velde ER, te Velde ER, Habbema JD, Fauser BC. Predictors of patients remaining anovulatory during clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. J Clin Endocrinol Metab 1998;83:2361–5.
- Rausch ME, Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, et al. Predictors of pregnancy in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2009;94:3458–66.
- Birdsall MA, Farquhar CM. Polycystic ovaries in pre and post-menopausal women. Clin Endocrinol (Oxf) 1996;44:269–76.
- Legro RS, Schlaff WD, Diamond MP, Coutifaris C, Casson PR, Brzyski RG, et al. Total testosterone assays in women with polycystic ovary syndrome: precision and correlation with hirsutism. J Clin Endocrinol Metab 2010;95:5305–13.
- Fenton A, Panay N. Management of polycystic ovary syndrome in postmenopausal women: a medical black hole. Climacteric 2008;11:89–90.
- Kassanos D, Trakakis E, Baltas CS, Papakonstantinou O, Simeonidis G, Salamalekis G, et al. Augmentation of cortical bone mineral density in women with polycystic ovary syndrome: a peripheral quantitative computed tomography (pQCT) study. Hum Reprod 2010;25:2107–14.
- Zborowski JV, Cauley JA, Talbott EO, Guzick DS, Winters SJ. Clinical review 116: bone mineral density, androgens, and the polycystic ovary: the complex and controversial issue of androgenic influence in female bone. J Clin Endocrinol Metab 2000;85:3496–506.
- Talbott E, Clerici A, Berga SL, Kuller L, Guzick D, Detre K, et al. Adverse lipid and coronary heart disease risk profiles in young women with polycystic ovary syndrome: results of a case-control study. J Clin Epidemiol 1998;51:415–22.
- Vgontzas AN, Legro RS, Bixler EO, Grayev A, Kales A, Chrousos GP. Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance. J Clin Endocrinol Metab 2001;86:517–20.
- Price VH, Roberts JL, Hordinsky M, Olsen EA, Savin R, Bergfeld W, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol 2000;43:768–76.
- Cheang KI, Nestler JE, Futterweit W. Risk of cardiovascular events in mothers of women with polycystic ovary syndrome. Endocr Pract 2008;14:1084–94.
- Taylor MC, Reema KA, Kunselman AR, Stetter CM, Dunaif A, Legro RS. Evidence for increased cardiovascular events in the fathers but not mothers of women with polycystic ovary syndrome. Hum Reprod. Published online April 19, 2011.
Practice Documents
ASRM Practice Documents have been developed to assist physicians with clinical decisions regarding the care of their patients.
Fertility preservation in patients with medical indications: a committee opinion (2026)
Expert guidelines on fertility preservation options—oocyte, embryo, ovarian and testicular tissue cryopreservation and counseling before gonadotoxic treatment.
The reproductive endocrinology and infertility subspecialist: definition, training, and scope of practice in the United States (2025)
Reproductive endocrinology and infertility (REI) subspecialists are physicians with extensive and specialized training in the diagnosis and treatment of complex reproductive disorders.
Improving access to care and delivery to marginalized and vulnerable populations: a committee opinion (2025)
ASRM committee opinion on reducing infertility care disparities, outlining barriers and actionable strategies to improve equitable access.
Evidence-based guideline: Premature Ovarian Insufficiency (2025)
This guideline on premature ovarian insufficiency (POI) offers best practice advice on the care of women with POI.Topic Resources
View more on the topic of polycystic ovary syndrome (PCOS)
Topic Resources
View more on the topic of hirsutism
Topic Resources
View more on the topic of contraception






